Abstract
Purpose of Review
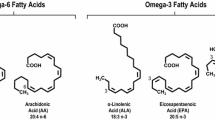
We discuss current controversies in the clinical use of omega-3 fatty acids (FA), primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and examine discrepancies between recent trials. Furthermore, we discuss potential side effects reported in these studies and the role of mixed omega-3 FA dietary supplements and concerns about their use.
Recent Findings
REDUCE-IT showed that addition of icosapent ethyl, a highly purified form of EPA, can reduce risk of cardiovascular events among statin-treated individuals with high triglycerides. Additional supportive evidence for EPA has come from other trials and meta-analyses of omega-3 FA therapy. In contrast, trials of mixed EPA/DHA products have consistently failed to improve cardiovascular outcomes. Discrepancies in results reported in RCTs could be explained by differences in omega-3 FA products, dosing, study populations, and study designs including the placebo control formulation. Evidence obtained from highly purified forms should not be extrapolated to other mixed formulations, including “over-the-counter” omega-3 supplements.
Summary
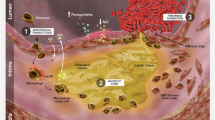
Targeting TG-rich lipoproteins represents a new frontier for mitigating ASCVD risk. Clinical and basic research evidence suggests that the use of omega-3 FA, specifically EPA, appears to slow atherosclerosis by reducing triglyceride-rich lipoproteins and/or inflammation, therefore addressing residual risk of clinical ASCVD.

Similar content being viewed by others
Change history
28 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11883-022-01043-5
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB et al. 2019 Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 380:11–22. REDUCE-IT showed that a highly purified form of EPA provided risk reduction compared to placebo among patients with relatively low or normal LDL-C.
Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol. 2016;67:22–7.
Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858:3131–40.
De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005;206:103–16.
Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res. 2016;167:257–80.
Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–79.
Welty FK, Schulte F, Alfaddagh A, Elajami TK, Bistrian BR, Hardt M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B4. FASEB J. 2021;35: e21448.
Dunbar RL, Nicholls SJ, Maki KC, Roth EM, Orloff DG, Curcio D, et al. Effects of omega-3 carboxylic acids on lipoprotein particles and other cardiovascular risk markers in high-risk statin-treated patients with residual hypertriglyceridemia: a randomized, controlled, double-blind trial. Lipids Health Dis. 2015;14:98.
Bays HE, Averna M, Majul C, Muller-Wieland D, De Pellegrin A, Giezek H, et al. Efficacy and safety of ezetimibe added to atorvastatin versus atorvastatin uptitration or switching to rosuvastatin in patients with primary hypercholesterolemia. Am J Cardiol. 2013;112:1885–95.
Sherratt SCR, Juliano RA, Mason RP. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim Biophys Acta Biomembr. 2020;1862: 183254.
Sherratt SCR, Mason RP. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun. 2018;496:335–8.
Mason RP, Jacob RF. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim Biophys Acta. 2015;1848:502–9.
Sherratt SCR, Dawoud H, Bhatt DL, Malinski T, Mason RP. Omega-3 and omega-6 fatty acids have distinct effects on endothelial fatty acid content and nitric oxide bioavailability. Prostaglandins Leukot Essent Fatty Acids. 2021;173: 102337.
Mason RP, Dawoud H, Jacob RF, Sherratt SCR, Malinski T. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother. 2018;103:1231–7.
Alfaddagh A, Elajami TK, Saleh M, Mohebali D, Bistrian BR, Welty FK. An omega-3 fatty acid plasma index >/=4% prevents progression of coronary artery plaque in patients with coronary artery disease on statin treatment. Atherosclerosis. 2019;285:153–62.
Budoff MJ, Muhlestein JB, Bhatt DL, Le Pa VT, May HT, Shaikh K et al 2021. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results. Cardiovasc Res. 117:1070–1077. EVAPORATE aimed to understand the effect of icosapent ethyl on plaque regression instead of hard outcomes.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y et al 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369:1090–8.
Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–50.
Albert CM, Cook NR, Pester J, Moorthy MV, Ridge C, Danik JS, Gencer B, Siddiqi HK, Ng C, Gibson H, Mora S, Buring JE, Manson JE. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. 2021;325:1061–73.
Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ et all 2020. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 324:2268–2280. STRENGTH used a different combination of omega-3 FA (both EPA and DHA) and was terminated early due to futility of benefit.
Doi T, Langsted A and Nordestgaard BG. 2021 A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. Eur Heart J. 42:4807–4817.
Olshansky B, Chung MK, Budoff MJ, Philip S, Jiao L, Doyle RT Jr, Copland C, Giaquinto A, Juliano RA, Bhatt DL. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur Heart J Suppl. 2020;22:J34–48.
Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H, Investigators O. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021;143:528–39.
Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine. 2021;38: 100997.
Adili R, Hawley M, Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018;139:10–8.
Gencer B, Djousse L, Al-Ramady OT, Cook NR, Manson JE, Albert CM. Effect of long-term marine-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation. 2021;144:1981–90.
Soong CS, Thompson JB, Poley JR, Hess DR. Hydroxy fatty acids in human diarrhea. Gastroenterology. 1972;63:748–57.
Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–74.
Cohen PA. Hazards of hindsight–monitoring the safety of nutritional supplements. N Engl J Med. 2014;370:1277–80.
Hilleman DE, Teply R, Packard KA. Knowledge, perceptions, and patterns of fish oil use in cardiac patients. J Pharm Pract. 2020;33:580–5.
Zargar A, Ito MK. Long chain omega-3 dietary supplements: a review of the National Library of Medicine Herbal Supplement Database. Metab Syndr Relat Disord. 2011;9:255–71.
Hilleman D, Smer A. Prescription omega-3 fatty acid products and dietary supplements are not interchangeable. Manag Care. 2016;25:46–52.
Mason RP, Sherratt SCR. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun. 2017;483:425–9.
Jackowski SA, Alvi AZ, Mirajkar A, Imani Z, Gamalevych Y, Shaikh NA, et al. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J Nutr Sci. 2015;4: e30.
Albert BB, Derraik JG, Cameron-Smith D, Hofman PL, Tumanov S, Villas-Boas SG, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015;5:7928.
Ritter JC, Budge SM, Jovica F. Quality analysis of commercial fish oil preparations. J Sci Food Agric. 2013;93:1935–9.
Nogueira MS, Kessuane MC, Lobo Ladd AA, Lobo Ladd FV, Cogliati B, Castro IA. Effect of long-term ingestion of weakly oxidised flaxseed oil on biomarkers of oxidative stress in LDL-receptor knockout mice. Br J Nutr. 2016;116:258–69.
Akesson A, Donat-Vargas C, Berglund M, Glynn A, Wolk A, Kippler M. Dietary exposure to polychlorinated biphenyls and risk of heart failure - a population-based prospective cohort study. Environ Int. 2019;126:1–6.
Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lepine MC, et al. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA (ComparED) Study. Am J Clin Nutr. 2016;104:280–7.
Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–59.
Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–83.
Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94.
Miyoshi T, Noda Y, Ohno Y, Sugiyama H, Oe H, Nakamura K, et al. Omega-3 fatty acids improve postprandial lipemia and associated endothelial dysfunction in healthy individuals - a randomized cross-over trial. Biomed Pharmacother. 2014;68:1071–7.
Schirmer SH, Werner CM, Binder SB, Faas ME, Custodis F, Bohm M, et al. Effects of omega-3 fatty acids on postprandial triglycerides and monocyte activation. Atherosclerosis. 2012;225:166–72.
Shearer GC, Savinova OV, Harris WS. Fish oil – how does it reduce plasma triglycerides? Biochim Biophys Acta. 2012;1821:843–51.
Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988;29:1451–60.
Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–806.
Kim CW, Addy C, Kusunoki J, Anderson NN, Deja S, Fu X, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:576.
Tanaka N, Zhang X, Sugiyama E, Kono H, Horiuchi A, Nakajima T, et al. Eicosapentaenoic acid improves hepatic steatosis independent of PPARalpha activation through inhibition of SREBP-1 maturation in mice. Biochem Pharmacol. 2010;80:1601–12.
Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res. 2009;50:412–23.
Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001;276:4365–72.
Botolin D, Wang Y, Christian B, Jump DB. Docosahexaneoic acid (22:6, n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J Lipid Res. 2006;47:181–92.
Xu J, Teran-Garcia M, Park JH, Nakamura MT, Clarke SD. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem. 2001;276:9800–7.
Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–22.
Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-talk between PPARgamma and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009;2009: 818945.
Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–4.
Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, et al. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol. 2010;325:54–63.
Rudkowska I, Caron-Dorval D, Verreault M, Couture P, Deshaies Y, Barbier O, et al. PPARalpha L162V polymorphism alters the potential of n-3 fatty acids to increase lipoprotein lipase activity. Mol Nutr Food Res. 2010;54:543–50.
Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, et al. Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res. 2002;43:979–85.
Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–93.
Miller M, Motevalli M, Westphal D, Kwiterovich PO Jr. Incorporation of oleic acid and eicosapentaenoic acid into glycerolipids of cultured normal human fibroblasts. Lipids. 1993;28:1–5.
Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, et al. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci U S A. 2008;105:5862–7.
Maitin V, Andreo U, Guo L, Fisher EA. Docosahexaenoic acid impairs the maturation of very low density lipoproteins in rat hepatic cells. J Lipid Res. 2014;55:75–84.
Wang H, Chen X, Fisher EA. N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J Clin Invest. 1993;91:1380–9.
Morton AM, Furtado JD, Lee J, Amerine W, Davidson MH, Sacks FM. The effect of omega-3 carboxylic acids on apolipoprotein CIII-containing lipoproteins in severe hypertriglyceridemia. J Clin Lipidol. 2016;10(1442–1451): e4.
Kris-Etherton PM, Harris WS, Appel LJ and American Heart Association. 2002 Nutrition C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 106:2747–57.
Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK et al. 2019 Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 140:e673-e691.
Rimm EB, Appel LJ, Chiuve SE, Djousse L, Engler MB, Kris-Etherton PM et al. 2018 Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 138:e35-e47.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646.
O’Keefe JH, Torres-Acosta N, O’Keefe EL, Saeed IM, Lavie CJ, Smith SE, et al. A Pesco-Mediterranean diet with intermittent fasting: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1484–93.
Virani SS, Morris PB, Agarwala A, Ballantyne CM, Birtcher KK, Kris-Etherton PM, et al. 2021 ACC Expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;78:960–93.
Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE et al. 2020 Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 141:e779-e806.
Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019;13:860–72.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;2020(41):111–88.
Sharma G, Martin SS, Blumenthal RS. Effects of omega-3 fatty acids on major adverse cardiovascular events: what matters most: the drug, the dose, or the placebo? JAMA. 2020;324:2262–4.
Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Addendum. 2021 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care 44(Suppl. 1):S125-S150. Diabetes Care. 2021;44:2183–2185.
Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107–39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Under a license agreement between Corrie Health and the Johns Hopkins University, the University owns equity in Corrie Health and the University, Dr. Marvel and Dr. Martin are entitled to royalty distributions. Additionally, Dr. Marvel and Dr. Martin are co-founders of and hold equity in Corrie Health. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Marvel has received research support from Apple and iHealth.
Dr. Martin also reports personal fees from Amgen, AstraZeneca, Esperion, 89bio, Sanofi-Aventis, Novo Nordisk, iHealth, Novartis, and DalCor; non-financial support from Apple and iHealth; grants and non-financial support from Google; and grants from Maryland Innovation Initiative, American Heart Association, Aetna Foundation, PJ Schafer Memorial Fund, David and June Trone Family Foundation, National Institutes of Health, and FH Foundation, outside the submitted work.
The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiometabolic Disease and Treatment.
The original online version of this article was revised:the author name Abdulhamied Alfaddagh was incorrectly written as Abdulhamied Al Faddagh.
Rights and permissions
About this article
Cite this article
Quispe, R., Alfaddagh, A., Kazzi, B. et al. Controversies in the Use of Omega-3 Fatty Acids to Prevent Atherosclerosis. Curr Atheroscler Rep 24, 571–581 (2022). https://doi.org/10.1007/s11883-022-01031-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01031-9