Abstract
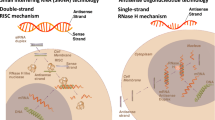
Antisense oligonucleotide therapy is a promising approach for the treatment of a broad variety of medical conditions. It functions at the cellular level by interfering with RNA function, often leading to degradation of specifically targeted abnormal gene products implicated in the disease process. Mipomersen is a novel antisense oligonucleotide directed at apolipoprotein (apoB)-100, the primary apolipoprotein associated with low-density lipoprotein cholesterol (LDL-C), which has recently been approved for the treatment of familial hypercholesterolemia. A number of clinical studies have demonstrated its efficacy in lowering LDL-C and apoB levels in patients with elevated LDL-C despite maximal medical therapy using conventional lipid-lowering agents. This review outlines the risks and benefits of therapy and provides recommendations on the use of mipomersen.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as •• Of major importance
Sniderman AD, Tsimikas S, Fazio S. The severe hypercholesterolemia phenotype: clinical diagnosis, management, and emerging therapies. J Am Coll Cardiol. 2014;63(19):1935–47.
Ito MK, McGowan MP, Moriarty PM. National Lipid Association Expert Panel on Familial H. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S38–45.
Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S1–8.
Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34(13):962–71.
Santos RD. Lipoprotein(a) and cardiovascular disease in heterozygous familial hypercholesterolemia: should we also blame the LDL receptor? J Am Coll Cardiol. 2014;63(19):1990–1.
Virani SS. What is new in the 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic risk in adults? Tex Heart Inst J Tex Heart Inst St Luke’s Episcopal Hosp Tex Child Hosp. 2014;41(3):304–5.
Mabuchi H, Koizumi J, Shimizu M, et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FH-LDL-Apheresis Study Group. Am J Cardiol. 1998;82(12):1489–95.
Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356(2):148–56.
Qian YW, Schmidt RJ, Zhang Y, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res. 2007;48(7):1488–98.
Kastelein JJ, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114(16):1729–35.
Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93.
Chi KN, Eisenhauer E, Fazli L, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2′-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst. 2005;97(17):1287–96.
Hooper AJ, Burnett JR. Update on primary hypobetalipoproteinemia. Curr Atheroscler Rep. 2014;16(7):423.
Ricotta DN, Frishman W. Mipomersen: a safe and effective antisense therapy adjunct to statins in patients with hypercholesterolemia. Cardiol Rev. 2012;20(2):90–5.
Wong E, Goldberg T. Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P T Peer-Rev J Formul Manag. 2014;39(2):119–22.
Crooke RM, Graham MJ, Lemonidis KM, Whipple CP, Koo S, Perera RJ. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J Lipid Res. 2005;46(5):872–84.
Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118(7):743–53.
Mullick AE, Fu W, Graham MJ, et al. Antisense oligonucleotide reduction of apoB-ameliorated atherosclerosis in LDL receptor-deficient mice. J Lipid Res. 2011;52(5):885–96.
Akdim F, Stroes ES, Sijbrands EJ, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55(15):1611–8.
Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105(10):1413–9.
Akdim F, Tribble DL, Flaim JD, et al. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011;32(21):2650–9.
Stein EA. Other therapies for reducing low-density lipoprotein cholesterol: medications in development. Endocrinol Metab Clin N Am. 2009;38(1):99–119.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006.
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet (1994) Nov 19;344(8934):1383–1389.
Heart Protection Study Collaborative G MRC/BHF Heart Protection. Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33.
McGowan MP, Tardif JC, Ceska R, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7(11):e49006. This study is an important Phase III study that showed significant reductions in LDL-C and apoB levels in patients with severe hypercholesterolemia on maximal lipid lowering therapy.
Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126(19):2283–92. This phase III study demonstrated significant reductions in LDL-C and apoB levels in patients with heterozygous FH on maximally tolerated statin therapy.
Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013;62(23):2178–84. This is a significant phase III study in patients with baseline LDL-C ≥100 with or at high risk for CHD on maximal lipid- lowering therapy that demonstrated significant reductions in LDL-C and apoB levels.
Flaim JD, Grundy JS, Baker BF, McGowan MP, Kastelein JJ. Changes in mipomersen dosing regimen provide similar exposure with improved tolerability in randomized placebo-controlled study of healthy volunteers. J Am Heart Assoc. 2014;3(2):e000560.
Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129(9):1022–32.
Davis KA, Miyares MA. Lomitapide: a novel agent for the treatment of homozygous familial hypercholesterolemia. Am J Health Syst Pharm: AJHP: Off J Am Soc Health Syst Pharmacists. 2014;71(12):1001–8.
Li Z, Hard ML, Grundy JS, Singh T, von Moltke LL, Boltje I. Lack of clinical pharmacodynamic and pharmacokinetic drug-drug interactions between warfarin and the antisense oligonucleotide mipomersen. Journal of cardiovascular pharmacology.2014 Mar 31.
Sinha G. Antisense battles small molecule for slice of rare lipid disorder market. Nat Biotechnol. 2013;31(3):179–80.
Rytelewski M, Tong JG, Buensuceso A, et al. BRCA2 inhibition enhances cisplatin-mediated alterations in tumor cell proliferation, metabolism, and metastasis. Molecular oncology. 2014. Jun 6 .
Culig Z. Targeting the androgen receptor in prostate cancer. Expert Opin Pharmacother. 2014;15(10):1427–37.
Magen I, Hornstein E. Oligonucleotide-based therapy for neurodegenerative diseases. Brain research. 2014. Apr 12.
Oustric V, Manceau H, Ducamp S, et al. Antisense oligonucleotide-based therapy in human erythropoietic protoporphyria. Am J Hum Genet. 2014;94(4):611–7.
Seto JT, Bengtsson NE, Chamberlain JS. Therapy of genetic disorders-novel therapies for Duchenne muscular dystrophy. Curr Pediatr Rep. 2014;2(2):102–12.
Gao X, Zhao J, Han G, et al. Effective dystrophin restoration by a novel muscle-homing peptide-morpholino conjugate in dystrophin-deficient mdx mice. Mol Ther: J Am Soc Gene Ther. 2014;22(7):1333–41.
Osman EY, Miller MR, Robbins KL, et al. Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models. Human molecular genetics. 2014. Apr 29.
Lieberman AP, Yu Z, Murray S, et al. Peripheral androgen receptor gene suppression rescues disease in mouse models of spinal and bulbar muscular atrophy. Cell Rep. 2014;7(3):774–84.
Liu J, Zhou Z, Li K, Han M, Yang J, Wang S. In vitro and in vivo protection against enterovirus 71 by an antisense phosphorothioate oligonucleotide. Archives of virology. 2014. Apr 23 .
Hashimoto M, Nara T, Hirawake H, Morales J, Enomoto M, Mikoshiba K. Antisense oligonucleotides targeting parasite inositol 1,4,5-trisphosphate receptor inhibits mammalian host cell invasion by Trypanosoma cruzi. Sci Rep. 2014;4:4231.
Lee CH, Kim JH, Lee SW. Prospects for nucleic acid-based therapeutics against hepatitis C virus. World J Gastroenterol: WJG. 2013;19(47):8949–62.
Schmidt PJ, Fleming MD. Modulation of hepcidin as therapy for primary and secondary iron overload disorders: preclinical models and approaches. Hematol/Oncol Clin N Am. 2014;28(2):387–401.
Compliance with Ethics Guidelines
Conflict of Interest
Anandita Agarwala declares no conflict of interest.
Peter Jones received personal fees for serving on the scientific advisory board for Merck and Amgen and the medical advisory board for Atherotech. He is the Chief Science Officer for the National Lipid Association.
Vijay Nambi has a provisional patent on the use of biomarkers in heart failure with Roche; research collaboration with GE; received personal fees from Sanofi for serving on the regional advisory board; received grants from Gillson Longenbaugh Foundation, Gulf Coast Regional Foundation, Methodist Hospital Research institute, and NIH/ NHLBI K23; national monitor for Anthera and research collaboration with Tomtec.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on New Drugs Approved for Homozygous FH
Rights and permissions
About this article
Cite this article
Agarwala, A., Jones, P. & Nambi, V. The Role of Antisense Oligonucleotide Therapy in Patients with Familial Hypercholesterolemia: Risks, Benefits, and Management Recommendations. Curr Atheroscler Rep 17, 467 (2015). https://doi.org/10.1007/s11883-014-0467-4
Published:
DOI: https://doi.org/10.1007/s11883-014-0467-4