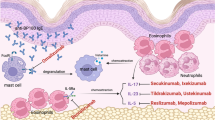
Abstract
Sublingual immunotherapy (SLIT) is a well-established allergen-specific immunotherapy and a safe and effective strategy to reorient inappropriate immune responses in allergic patients. SLIT takes advantage of the tolerogenic environment of the oral mucosa to promote tolerance to the allergen. Several clinical studies have investigated the complex interplay of innate and adaptive immune responses that SLIT exploits. The oral immune system is composed of tolerogenic dendritic cells that, following uptake of allergen during SLIT, support the differentiation of T helper cell type 1 (Th1) and the induction of IL-10-producing regulatory T cells. Following SLIT, allergic disease-promoting T helper cell type 2 (Th2) responses shift to a Th1 inflammatory response, and IL-10 and transforming growth factor (TGF)-β production by regulatory T cells and tolerogenic dendritic cells suppress allergen-specific T cell responses. These immune changes occur both in the sublingual mucosa and in the periphery of a patient following SLIT. SLIT also promotes the synthesis of allergen-specific IgG and IgA antibodies that block allergen-IgE complex formation and binding to inflammatory cells, thus encouraging an anti-inflammatory environment. Several of these revealing findings have also paved the way for the identification of biomarkers of the clinical efficacy of SLIT. This review presents the emerging elucidation of the immune mechanisms mediated by SLIT.
Similar content being viewed by others
Abbreviations
- SLIT:
-
Sublingual immunotherapy
- SCIT:
-
Subcutaneous immunotherapy
- OIT:
-
Oral immunotherapy
- mDC:
-
Myeloid dendritic cell
- pDC:
-
Plasmacytoid dendritic cell
- oLC:
-
Oral Langerhans cell
- Treg:
-
Regulatory T cell
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Makatsori M, Scadding GW, Lombardo C, Bisoffi G, Ridolo E, Durham SR, et al. Dropouts in sublingual allergen immunotherapy trials—a systematic review. Allergy. 2014;69:571–80.
Linkov G, Toskala E. Sublingual immunotherapy: what we can learn from the European experience. Curr Opin Otolaryngol Head Neck Surg. 2014;22:208–10.
Jutel M. Allergen-specific immunotherapy in asthma. Curr Treat Options Allergy. 2014;1:213–9.
Nelson HS. Sublingual immunotherapy: the U.S. experience. Curr Opin Allergy Clin Immunol. 2013;13:663–8.
Moran TP, Vickery BP, Burks AW. Oral and sublingual immunotherapy for food allergy: current progress and future directions. Curr Opin Immunol. 2013;25:781–7.
Sato S, Yanagida N, Ogura K, Imai T, Utsunomiya T, Iikura K, et al. Clinical studies in oral allergen-specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014;164:1–9.
Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–6.
Begin P, Chinthrajah RS, Nadeau KC. Oral immunotherapy for the treatment of food allergy. Hum Vaccines Immunotherapeutics. 2014;10:29–8.
Fujita H, Soyka M, Akdis M, Akdis C. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012;2:2.
Cavkaytar O, Akdis CA, Akdis M. Modulation of immune responses by immunotherapy in allergic diseases. Curr Opin Pharmacol. 2014;17:30–7.
Novak N, Gros E, Bieber T, Allam JP. Human skin and oral mucosal dendritic cells as ‘good guys’ and ‘bad guys’ in allergic immune responses. Clin Exp Immunol. 2010;161:28–33.
Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41.
Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–7.
Mutyambizi K, Berger CL, Edelson RL. The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci. 2009;66:831–40.
Allam J-P, Würtzen PA, Reinartz M, Winter J, Vrtala S, Chen K-W, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-β1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–45.
Angelini F, Pacciani V, Corrente S, Silenzi R, Di Pede A, Polito A, et al. Dendritic cells modification during sublingual immunotherapy in children with allergic symptoms to house dust mites. World J Pediatr. 2011;7:24–30.
Zimmer A, Bouley J, Le Mignon M, Pliquet E, Horiot S, Turfkruyer M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1020–30.
Fujimura T, Yonekura S, Taniguchi Y, Horiguchi S, Saito A, Yasueda H, et al. The induced regulatory T cell level, defined as the proportion of IL-10(+)Foxp3(+) cells among CD25(+)CD4(+) leukocytes, is a potential therapeutic biomarker for sublingual immunotherapy: a preliminary report. Int Arch Allergy Immunol. 2010;153:378–87.
Nagai Y, Shiraishi D, Tanaka Y, Nagasawa Y, Ohwada S, Shimauchi H, et al. Transportation of sublingual antigens across sublingual ductal epithelial cells to the ductal antigen-presenting cells in mice. Clin Exp Allergy. 2014. doi:10.1111/cea.12329.
Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9.
Demoly P, Calderon MA. Dosing and efficacy in specific immunotherapy. Allergy. 2011;66:38–40.
Guida G, Boita M, Scirelli T, Bommarito L, Heffler E, Badiu I, et al. Innate and lymphocytic response of birch-allergic patients before and after sublingual immunotherapy. Allergy Asthma Proc. 2012;33:411–5.
Mascarell L, Lombardi V, Zimmer A, Louise A, Tourdot S, Van Overtvelt L, et al. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+ T cells. Clin Exp Allergy. 2009;39:1910–9.
Ciprandi G, Fenoglio D, Cirillo I, Vizzaccaro A, Ferrera A, Tosca MA, et al. Induction of interleukin 10 by sublingual immunotherapy for house dust mites: a preliminary report. Ann Allergy Asthma Immunol. 2005;95:38–44.
Burastero SE, Mistrello G, Falagiani P, Paolucci C, Breda D, Roncarolo D, et al. Effect of sublingual immunotherapy with grass monomeric allergoid on allergen-specific T-cell proliferation and interleukin 10 production. Ann Allergy Asthma Immunol. 2008;100:343–50.
Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13.
Bahceciler NN, Galip N. Comparing subcutaneous and sublingual immunotherapy: what do we know? Curr Opin Allergy Clin Immunol. 2012;12:640–7.
Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–23.
Pellerin L, Jenks J, Bégin P, Bacchetta R, Nadeau K. Regulatory T cells and their roles in immune dysregulation and allergy. Immunol Res. 2014;58:358–68.
Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106.
Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61.
Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6.
O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy. Am J Respir Crit Care Med. 2009;180:936–47.
Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40:922–32.
Yamanaka K-I, Yuta A, Kakeda M, Sasaki R, Kitagawa H, Gabazza EC, et al. Induction of IL-10-producing regulatory T cells with TCR diversity by epitope-specific immunotherapy in pollinosis. J Allergy Clin Immunol. 2009;124:842–5.
Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606.
Bonvalet M, Moussu H, Wambre E, Ricarte C, Horiot S, Rimaniol AC, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2012;42:1745–55. By using tetramers to observe allergen-specific T cell responses following SLIT, this study offers a thorough contradiction to previous findings that the changes in the induction of Tregs and the shifting of Th2 to Th1 responses directly correlated with the early clinical efficacy of SLIT.
Suárez-Fueyo A, Ramos T, Galán A, Jimeno L, Wurtzen PA, Marin A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133:130–8. This detailed longitudinal study of the systemic effects of SLIT clearly shows the early and late phases of immune modulations. These changes observed included the early exacerbation of Th2 responses and serum allergen-specific IgE, followed by the later shift of Th2 to Th1 responses and increased blocking allergen-specific IgG4 as well as the induction of Tregs.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500.
Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608.
Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–35.
Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215–24. This study clearly shows how following SLIT, epigenetic regulation of the Foxp3 locus through methylation of CpG sites determines the stability and function of induced regulatory T cells, and that this may be a new biomarker for monitoring the clinical efficacy of SLIT.
Syed A, Garcia MA, Lyu S-C, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. 2014;133:500–10.
James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–16.
Queirós MGJ, Silva DAO, Siman IL, Ynoue LH, Araújo NS, Pereira FL, et al. Modulation of mucosal/systemic antibody response after sublingual immunotherapy in mite-allergic children. Pediatr Allergy Immunol. 2013;24:752–61.
Ott H, Sieber J, Brehler R, Fölster-Holst R, Kapp A, Klimek L, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:1394–401.
Wahn U, Klimek L, Ploszczuk A, Adelt T, Sandner B, Trebas-Pietras E, et al. High-dose sublingual immunotherapy with single-dose aqueous grass pollen extract in children is effective and safe: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 2012;130:886–93.
Creticos PS, Esch RE, Couroux P, Gentile D, D’Angelo P, Whitlow B, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–8.
Durham SR, GT-08 investigators. Sustained effects of grass pollen AIT. Allergy. 2011;66:50–2.
Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, et al. Development of cockroach immunotherapy by the inner-city asthma consortium. J Allergy Clin Immunol. 2014;133:846–52.
Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740–52.
Fernández-Rivas M, Garrido Fernández S, Nadal JA, Alonso Díaz De Durana MD, García BE, González-Mancebo E, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64:876–83.
Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55.
Baron-Bodo V, Horiot S, Lautrette A, Chabre H, Drucbert AS, Danzé PM, et al. Heterogeneity of antibody responses among clinical responders during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2013;43:1362–73.
Pereira C, Bartolome B, Asturias JA, Ibarrola I, Tavares B, Loureiro G, et al. Specific sublingual immunotherapy with peach LTP (Pru p 3). One year treatment: a case report. Cases J. 2009;2:6553.
Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–6.
Gloudemans AK, Lambrecht BN, Smits HH. Potential of immunoglobulin a to prevent allergic asthma. Clin Dev Immunol. 2013;2013:12.
D.O. Miranda, D.A.O. Silva, J.F.C. Fernandes, Queirós, M.G.J., H.F. Chiba, L.H. Ynoue, R.O. Resende, J.D.O. Pena, S.-S.J. Sung, G.R.S. Segundo, E.A. Taketomi (2011) Serum and salivary IgE, IgA, and IgG4 antibodies to Dermatophagoides pteronyssinus and its major allergens, Der p1 and Der p2, in allergic and nonallergic children. Clin Dev Immunol 2011:11.
Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1159–62.
Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125:660–6.
Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–34.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
David C. Jay and Kari C. Nadeau declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Immunotherapy and Immunomodulators
Rights and permissions
About this article
Cite this article
Jay, D.C., Nadeau, K.C. Immune Mechanisms of Sublingual Immunotherapy. Curr Allergy Asthma Rep 14, 473 (2014). https://doi.org/10.1007/s11882-014-0473-1
Published:
DOI: https://doi.org/10.1007/s11882-014-0473-1