Abstract
Air pollution is a significant health threat around the world. Young children are a more vulnerable population. Environmental Protection Agency (EPA) and World Health Organizations (WHO) guidelines may not adequately protect them. Given children’s rapid growth, it is important to review studies, consider the weight of evidence, and identify knowledge gaps. Our purpose was to conduct a systematic literature review of epidemiological studies of adverse health outcomes not previously considered; specifically, we reviewed evidence for traffic-related air pollution (TRAP) CO, NO2, SO2, O3, PM10, and PM2.5 in children ≤ 5 years in age, with special attention to adverse effects occurring within current air pollutant guidelines. Data sources are electronic search of PubMed, MEDLINE, and Google Scholar. Articles on air pollutants and additional health outcomes in children (0–5 years), between Jan. 1997 and Dec. 2018. Search included terms such as “air pollution, children, and adverse effects”. Fifty-two studies met the inclusion criteria. Seven (7) health outcome categories were identified: respiratory diseases; developmental disorders; allergies, eczema, and allergic rhinitis; ear infections; cancer; obesity; and others, with greatest associations for respiratory and developmental disorders. Strongest findings were for NO2, O3, PM10, and PM2.5, and most health effects reviewed occurred within WHO limits. Our findings are pertinent for health professionals, researchers, government officials, and others to collaboratively support policy efforts toward exposure reductions for pregnant women and children to prevent acute and chronic diseases. This is critical for building a culture of health and ensuring health equity for vulnerable populations.
Similar content being viewed by others
Introduction
Traffic-related air pollution (TRAP) is a global health threat. Both the World Health Organization (WHO) and the United States Environmental Protection Agency (EPA) have developed guidelines (Table 1) for a number of pollutants: nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), and particulate matter (PM10, PM2.5) (WHO 2005; EPA 2016). The criteria for acceptable levels of these air pollutants are set to protect the “public health with a margin of safety”. However, there may be sensitive subpopulations for which these standards are not adequate.
One potential subpopulation are children within the first 5 years of life. There is increasing evidence that the timing of environmental exposures plays a critical role in influencing healthy biological development and gene expression (Fox et al. 2010). Further supporting this notion, a global report by the WHO stated the “per capita number of healthy life years lost [globally] to environmental risk factors was about 5-times greater in children under five years of age than in the total population” (WHO 2005).
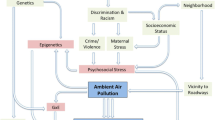
The biological mechanisms through which air pollutants promote adverse health effects, especially with regard to young children, are not fully understood and are likely disease-specific and multifactorial. Some of the diseases mentioned in this literature review, such as asthma and other atopic diseases, have a genetic component; however, TRAP may augment the effects of these diseases. One review article suggests oxidative stress may be the primary, underlying mechanism responsible for the harmful effects of TRAP (Salvi 2007). The reactive, oxidative species produced in response to air pollutants can overwhelm the redox system and damage the cell wall, lipids, proteins, and DNA, leading to airway inflammation and hyper-reactivity (Kim and Hong 2012). Pollutants may also cause harmful effects through epigenetic alterations, which can control the expression of genes without changing the DNA sequence. TRAP may contribute to an inflammatory response that could lead to initiation and/or progression of disease through inflammatory cascade. Inhalation of TRAP has been found associated with pulmonary inflammatory responses that may trigger a cascade of events, including subsequent release of pro-thrombotic and anti-inflammatory cytokines, setting a systemic inflammatory process in motion (Vadillo-Ortega et al. 2014). Exposure to air pollution may potentially promote neuroinflammation, the immune response of the brain, and accumulation of Aβ42 and α-synuclein starting in childhood (Calderón-Garcidueñas et al. 2008). This inflammation process can lead to disruption of the blood–brain barrier (BBB). Because of the disruption of the BBB, exposure to air pollution should be considered a risk factor for central nervous system disorders still to be identified for both children and adults.
Aside from genetic components, exposure to TRAP during pregnancy could potentially lead to adverse impact on the placent and affecting birth weight, which is associated with poor health outcomes over the course of a lifetime (e Silva et al. 2008). If the placenta becomes altered by TRAP, it can reduce the amount of nutrition and/or oxygen provided to the developing child and/or reduce the function of the barrier against toxic agents within the womb (Maisonet et al. 2004). Additional research assessing biological mechanisms for each pollutant and for differing pediatric diseases is needed.
Given the potential vulnerability of early childhood, it is important to review studies and consider the weight of the evidence. A number of systematic reviews have assessed adverse health outcomes around the time of birth (birth defects, sudden infant death syndrome, intrauterine growth retardation, and low birth weight) in association with TRAP (Glinianaia et al. 2004; Šrám et al. 2005). The purpose of this systematic review is to assess for additional adverse health outcomes associated with TRAP exposures among children ages 0–5 years, identify critical exposure windows, and assess the potential effectiveness of air quality guidelines.
Methods
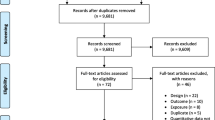
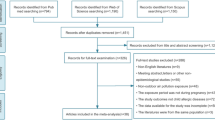
A systematic literature review was conducted by our team to identify epidemiological studies pertaining to TRAP and health outcomes in children (Fig. 1). Studies were identified from PUBMED, Google Scholar, and MEDLINE databases using primary search terms: “air pollution/traffic exposure” and “children/pregnancy/maternal exposure” combined with the following terms: diabetes, atopic disease, allergies, autism, attention deficit hyperactivity disorder (ADHD), respiratory, asthma, obesity, eczema, endocrine, cancer, diabetes, otitis media, and adverse effects. Our search resulted in 2384 studies which were further screened based on the criteria listed below (Fig. 2).
Inclusion criteria
Peer-reviewed, epidemiological studies on health effects of criteria pollutants (CO, NO2, SO2, O3, PM10, PM2.5) in exposed children, infants, or fetuses with health outcomes assessed within the first 5 years of life. Articles from the years January 1, 1997, to December 30, 2018, were included.
Exclusion criteria
Studies focused on congenital birth defects, adverse birth outcomes (IUGR, low birth weight, preterm delivery), and infant mortality and studies focusing on indoor air pollutants were excluded. Additionally, studies with older children (> 5 years of age) or studies which included a range of children in which findings were not specific for children ≤ 5 years in age were excluded.
Air quality guideline comparison
Exposures were compared with current WHO limits to determine if the exposure was above or below the guidelines (Table 1). For CO and O3, the majority of the exposures were not based on an 8-h time frame and thus were not directly comparable to guidelines. For comparison, we assessed if the annual averages based on 24-h mean exposures were within the 8-h mean guideline and compared with the WHO limits for O3 and EPA for CO.
Results
Fifty-two studies (n = 52) were identified and separated into the following health outcome categories: respiratory diseases, developmental disorders, allergies/eczema/allergic rhinitis, ear infections, cancer, obesity, and others (Table 2). The four windows of exposure categories identified and summarized were pregnancy, birth to 1 year, 1 to 5 years, and birth to 5 years. Figures 3 and 4 summarize the results.
Disease category with total number of studies, studies with significant findings, and studies within guidelines. *Majority of the studies with exposures for CO and O3 were not directly comparable to guidelines, as they did not assign exposure based on an 8-h timeframe, but used more annual averages based on 24-h exposures. Thus, our comparison with the guidelines is based on annual exposures
Disease category with total number of studies, studies with significant findings, and studies within guidelines across exposure windows. *Majority of the studies with exposures for CO and O3 were not directly comparable to guidelines, as they did not assign exposure based on an 8-h timeframe, but used more annual averages based on 24-h exposures. Thus, our comparison with the guidelines is based on annual exposures
Respiratory diseases
Twenty-eight (n = 28) studies (Table 2) assessed TRAP exposures and respiratory diseases, with the majority focused on asthma (Aguilera et al. 2012; Bai et al. 2018; Braga et al. 2001; Brauer et al. 2007, 2002; Clark et al. 2010; Deng et al. 2016; Gehring et al. 2002, 2015; Gruzieva et al. 2013; Hertz-Picciotto et al. 2007; Horne et al. 2018; Jedrychowski et al. 2010, 2013; Karr et al. 2005, 2009; Kennedy et al. 2018; MacIntyre et al. 2013; Mölter et al. 2015; Morales et al. 2015; Nastos et al. 2010; Nordling et al. 2008; Rodríguez-Villamizar et al. 2018; Sbihi et al. 2016; Song et al. 2018; Stern et al. 2013; Yang et al. 2004; Zhu et al. 2017). The other respiratory health endpoints studied included pneumonia, bronchitis, wheezing, dry cough, croup, acute lower respiratory infections, flu/severe cold, and lung function assessed with spirometry. Across the studies, the health endpoints were determined in a variety of ways including self-report by a parent of a doctor diagnosis, symptom questionnaires completed by a parent, physician diagnosis, laboratory and respiratory tests, emergency room visits, and hospital admissions. Seventeen studies (61%) included a cohort design while the others were case–control, time series, and cross-sectional. Adjustment for confounders varied between the study types with cohort studies adjusting for more confounders, though only 12 (71%) of the cohort studies adjusted for the potential confounding by exposure to environmental tobacco smoke. All six TRAP pollutants were studied, and the majority assessed NO2. Of the significant findings for CO, NO2, PM10, and SO2, results revealed that at least half or more were within the WHO limits. All of the significant studies for the O3 pollutant had average exposures within WHO limits. The strongest findings were for an odds ratio (OR) of 4.06 (95% CI: 1.93–8.57) for pneumonia in the first year of life associated with PM2.5, identified through a pooling data cohort study of 15,980 infants in Europe (the ESCAPE Project) and average PM2.5 exposures were within WHO limits (MacIntyre et al. 2013). The ESCAPE Project is a large, pooled study of 10 European birth cohorts where parent reports physician-diagnosed pneumonia, otitis media, and croup during early childhood and was assessed in relation to annual average pollutant levels of NO2, PM10, and PM2.5.
For the respiratory disease category, all four windows of exposure have been studied. Among the significant studies within each exposure window, at least one pollutant had 100% of its study findings within the WHO limits (window and pollutant): pregnancy (PM10), birth to 1 year (O3, SO2), 1 to 5 years (CO, NO2, O3), and birth to 5 years (O3). The respiratory category is well researched with 10 or more studies conducted for CO, NO2, PM10, and PM2.5 pollutants, but more research is needed to better assess O3 and SO2. One of the cohort studies followed 505 pregnant women and their neonates followed from pregnancy through 5 years of age living in Krakow, Poland (Jedrychowski et al. 2010). Pregnant women were recruited to take part, and within the second trimester, their PM2.5 exposure was assessed through a personal monitoring device. After delivery, participants were followed up at their homes to record children’s respiratory symptoms every 3 months in the child’s first 2 years of life and every 6 months later. In the fifth year, researchers assessed lung function with spirometry and found significant lung dysfunction in the child associated with PM2.5 exposure of a mother during pregnancy and average pollutant exposures were within guidelines. In a cohort study (> 37,400 children born in British Columbia), researchers found a significant relationship between early life (within utero and first year of life) exposure to CO, NO2, PM10, and SO2 and increased risk of asthma diagnosis. Within this study, researchers used a nested case–control design with asthma cases identified through outpatient and hospitalization records. Asthma cases were age- and sex-matched to five randomly chosen controls from the eligible cohort. The mean (± SD) age at end of follow-up was 48 ± 7 months and ranged from 36 to 59 months. The findings indicated significant risks within this study across both in utero and first-year-of-life exposures, though the first-year-of-life exposures were associated with stronger point estimates. Furthermore, results for this study showed that average exposure to NO2, PM10, and SO2 were all within WHO limits (Clark et al. 2010).
Developmental disorders
Eight (n = 8) studies assessed TRAP exposures and developmental disorders, including autism and additional mental challenges (i.e., slower reaction time) (Becerra et al. 2013; Guxens et al. 2011; Kerin et al. 2018; Kim et al. 2014; Lin et al. 2014; Raz et al. 2014; Sentís et al. 2017; Volk et al. 2013). Development disorders were identified based on the Bayley Scales of Infant Development Assessment as well as by self-report by a parent of a physician diagnosis for developmental disorder. Four of the 8 studies have included a cohort design, with remaining 4 case–control studies. All six TRAP pollutants were studied, and most assessed NO2. All of the significant studies for four out of the six pollutants assessed (CO, NO2, O3, SO2) had average exposures within the WHO limits. The study with the strongest findings was the CHARGE study and found that children with autism were more likely to live in the highest quartile of TRAP exposures during the first year of life (OR = 3.10, 95% CI: 1.76–5.57) compared to controls and higher NO2 during pregnancy was associated with greater decreases in cognitive scores (Volk et al. 2013). CHARGE is a case–control study conducted among 279 preschool children with autism and 245 matched controls living within California. Potential participants were identified through the California Department of Developmental Services (DDS) and its regional centers that coordinate services for children with autism and developmental delay. Air pollutant exposures were assigned based on a parent-reported residential history. In a California population-based case–control study (> 7600 children 3–5 years in age), researchers found an association between autism diagnosis and O3 (OR = 1.12, 95%CI: 1.06–1.19) and PM2.5 (OR = 1.15, 95%CI: 1.06–1.24). Furthermore, among parents with a high school education, a significant association was identified for NO2, O3, PM10, and PM2.5. Altogether CO, O3, and NO2 average exposures were within WHO limits, though particulate matter (PM10 and PM2.5) was above (Becerra et al. 2013).
Two windows of exposure have been studied, pregnancy and birth to 1 year, and all six TRAP pollutants were studied for both windows. For both exposure windows, two pollutants had all of their significant findings within WHO limits (window and pollutant): pregnancy (CO, SO2) and birth to 1 year (NO2, SO2). Furthermore, NO2 and SO2 showed consistency across both exposure windows. Within the CHARGE study, the findings revealed an association between autism and exposures within the first year of life including NO2 (OR = 2.06, 95%CI: 1.37–3.09), PM2.5 (OR = 2.12, 95%C CI: 1.45–3.10), and PM10 (OR = 2.14, 95% CI: 1.46–3.12), and no association found for O3 (Volk et al. 2013). Within a second cohort study of 1298 children living within various regions of Spain (the INMA-Environment and Childhood-Project is a population birth cohort assessing a range of health outcomes), researchers identified a significant association for NO2 and impaired reaction time (Sentís et al. 2017). Within this INMA project, researchers estimated prenatal and postnatal NO2 levels for all the participants’ residential addresses. Then, researchers assessed attentional function using the Kiddie-Conners Continuous Performance Test (K-CPT) and tested children at 4 and 5 years of age. Researchers found that prenatal exposure to NO2 was associated with an impaired standard error of the hit reaction time (increase of 1.12 ms [95% CI; 0.22 a 2.02] per 10 μg/m3 increase NO2) and increased omission errors (6% [95% CI; 1.01 to 1.11] per 10 μg/m3 increase NO2) and average exposures were within WHO limits. Overall, more research is needed to confirm these trends as all pollutants except NO2 have had ten or fewer studies conducted for each.
Allergies, eczema, and rhinitis
Seven (n = 7) studies assessed TRAP exposure and allergies, eczema, or rhinitis and all studies have included a cohort design (Aguilera et al. 2012; Brauer et al. 2007, 2002; Deng et al. 2016; Huang et al. 2015; Kim et al. 2017; Lu et al. 2017). The allergy, eczema, and rhinitis health outcomes were identified through parent report of physician diagnosis, physician diagnosis, or a standardized questionnaire by the International Study of Asthma and Allergies in Childhood. Five out of the 7 studies included adjustment for potential confounding by environmental tobacco smoke and the majority of studies did include a wide range of potential confounding variables. All six TRAP pollutants were studied, with majority of studies assessing NO2. All of the significant studies for two out of six pollutants assessed (CO, O3) had average exposures within WHO limits. NO2 results revealed that among studies with significant findings, half had exposures within WHO limits. In a cohort study of 177 children in Korea (< 5 years of age), followed over 17 months, researchers found significant associations with atopic dermatitis symptoms and percent change symptoms associated with same-day pollutant exposures PM10 (3.2, 95%CI: 1.5–4.9), NO2 (5.0, 95%CI: 1.4–8.8), and O3 (6.1, 95%CI: 3.2–9.0). Both NO2 and O3 average exposures were within WHO limits (Kim et al. 2017). The strongest findings were found within a cohort study of 2598 children in Changsha, China, for allergic rhinitis associated with NO2 exposure within the third trimester of pregnancy (OR = 1.77, 95% CI: 1.09–2.89), and exposures were above WHO limits (Deng et al. 2016). However, within the INMA study, researchers did not find an association between NO2 and eczema (Aguilera et al. 2012).
Three windows of exposure have been studied, pregnancy, birth to 1 year, and 1 to 5 years, and only the birth to 1 year window had all six TRAP pollutants studied. Two exposure windows had a pollutant in which all of their significant findings were within the WHO limits (window and pollutant): birth to 1 year (NO2) and 1 to 5 years (NO2, O3). More research is needed to confirm these trends since all but NO2 have had five or fewer studies conducted.
Ear infections
Nine (n = 9) studies assessed TRAP exposures and ear infections with 7 of the studies of cohort design and the others were case-crossover studies (Aguilera et al. 2012; Brauer et al. 2007, 2002, 2006; Kennedy et al. 2018; MacIntyre et al. 2013, 2011; Kousha and Castner 2016; Zemek et al. 2010). Ear infection outcomes were assessed either by a parent reporting a doctor diagnosis (n = 5) or by medical record assessment and either reported as ear infection or as a combined ear/nose/throat infection. Majority of the studies included a lengthy list of confounders that were adjusted for and 6 of the 9 studies included adjustment for exposure to environmental tobacco smoke. All six TRAP pollutants were studied, most assessing NO2. All of the significant studies for one pollutant assessed (O3) had average exposures within the WHO limits. CO, NO2, and PM2.5 results revealed half or more of the significant studies had exposures within guidelines. In a case cross-over study of over 14,500 visits to emergency room for ear infections in Canadian children (1–3 years in age), researchers used emergency department visits for children (1–3 years in age) in Edmonton, in the province of Alberta, and assessed the potential association with TRAP (Zemek et al. 2010). In the Canadian study, researchers found significant associations for NO2 for all months (OR = 1.05, 95%CI: 1.01–1.08) and O3 in cold months (OR = 1.07, 95%CI: 1.01–1.14) associated, but no significant for CO (OR = 1.08, 95%CI: 1.00–1.16). Both O3 and CO were within WHO limits. In the comparison of all the studies included among this health outcome, the strongest findings were for ear/nose/throat infection and PM2.5 (OR = 1.20, 95% CI: 1.01–1.42) within a birth cohort study of 4146 children in the Netherlands, with average exposures above WHO limits (Brauer et al. 2002).
Three windows of exposure have been studied, pregnancy, birth to 1 year, and 1 to 5 years, and all six TRAP pollutants were studied for birth to 1 year and 1 to 5 years. Pollutants in both windows had at least one pollutant in which all of its significant studies had average exposures within the WHO limits (window and pollutant): pregnancy (NO2) and 1 to 5 years (CO, O3, PM2.5). Within the INMA Project, significant association between NO2 and ear infections was found for exposure during the first year of life (RR = 1.15, 95%CI: 1.01–1.31) and with average NO2 exposures within WHO limits (Aguilera et al. 2012). Overall, more research is needed to confirm these trends as all pollutants have had ten or fewer studies conducted.
Cancer
Four (n = 4) studies assessed TRAP exposures in relation to childhood cancers and included total cancer as well as for specific childhood cancers such as astrocytomas, acute lymphoblastic leukemia (ALL), teratomas, and retinoblastomas (Feychting et al. 1998; Ghosh et al. 2013; Heck et al. 2013; Lavigne et al. 2017). Two of the studies were cohort design and the other 2 were case–control. Adjustment for confounders differed across the studies, with one study reporting very few confounders (magnetic fields and socioeconomic status) and only one study reporting adjustment for environmental tobacco smoke exposure. Only three of the six TRAP pollutants were studied (CO, NO2, PM2.5), with majority assessing NO2. Two of the three pollutants assessed (CO, NO2) had average exposures within the WHO limits among their significant studies. PM2.5 results revealed that of the significant studies, half had exposures within guidelines. In a cohort study of 2,350,000 births in Ontario, Canada, researchers found PM2.5 exposure over the entire pregnancy associated with increased risk of astrocytoma (HR = 1.38, 95%CI: 1.01–1.88) and NO2 exposure in the first trimester associated with acute lymphoblastic leukemia (ALL) (HR = 1.20, 95%CI: 1.02–1.41). Both pollutant average exposures were within WHO limits (Lavigne et al. 2017). Data within this Canadian study were obtained from the Mother-Baby Linked database (MOMBABY), an administrative database linking the hospital admission records of delivering mothers and their newborns across Ontario. Subjects with cancer were identified through the childhood cancer cases from the Pediatric Oncology Group of Ontario Networked Information System (POGONIS) and linked with the MOMBABY database. This POGONIS is a population-based registry that prospectively captures all cases of pediatric cancer diagnosed and treated at one of the five tertiary pediatric oncology centers in Ontario. Researchers assigned air pollutant exposures, based on residential addresses. Among the four studies included, the strongest findings were found in a case–control study of 3590 children in California for PM2.5 (exposure during pregnancy) and retinoblastoma (OR = 1.57, 95% CI: 1.10–2.23), though average pollutant exposures were above the WHO limits (Heck et al. 2013). Within this California case–control study, the cases of cancer were identified through the California Cancer Registry. The California study included children 5 years of age or younger, identified in the California Cancer Registry (born 1998–2007) who could be linked to a California birth certificate (n = 3590). Controls were selected at random from California birthrolls (n = 80,224). Air pollution exposure was estimated based on each child’s address as listed on the birth certificate.
Three windows of exposures were studied, pregnancy, birth to 1 year, and 1 to 5 years. For two windows of exposures each had at least one pollutant with all their significant findings with average exposures within WHO limits (window and pollutant): pregnancy (CO, NO2) and birth to 1 year (CO). Within the California study described above, retinoblastoma was significantly associated with PM2.5 exposures during pregnancy, though average exposures were above the WHO limits. More research is needed to confirm these trends as not all TRAP pollutants were studied and all studied pollutants had five or fewer studies conducted.
Obesity
Three (n = 3) studies assessed TRAP exposure in relation to obesity and all studies have included a cohort design (Chiu et al. 2017; Fleisch et al. 2017; Kim et al. 2016). Obesity was assessed through height and weight measurements used to calculate body mass index (BMI) collected by trained personnel and additional waist circumference and skinfold thickness measurements were collected. Additionally, one study included bipolar bioelectric impedance to estimate fat mass and plasma serum measurements for leptin and adiponectin (Fleisch et al. 2017). Among all 3 studies, only particulate matter pollutants were assessed. None of the significant studies for either pollutant assessed (PM10, PM2.5) had average exposures within WHO limits. In a birth cohort study (239 children in Boston, Massachusetts, within the ACCESS Project), researchers found significant association with exposure to PM2.5 during pregnancy on waist-to-hip ratio among girls (cumulative effect = 0.023, 95%CI: 0.01–0.03), body mass index (BMI) score (cumulative effect = 0.21, 95%CI: 0.003–0.37), and fat mass (cumulative effect = 0.36, 95%CI: 0.12–0.68) in boys between 4 and 5 years of age. Pollutant exposures for PM2.5 overlapped with children below and above WHO limits (Chiu et al. 2017). Within the ACCESS Project, participants were from the Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, a pregnancy cohort originally funded to recruit 500 mother–child pairs to examine the effects of early life exposures on childhood respiratory health. Between August 2002 and January 2007, English- or Spanish-speaking pregnant women (≥ 18 years old) receiving care at Brigham & Women’s Hospital (BWH), Boston Medical Center (BMC), and affiliated community health centers were enrolled (at 28.4 ± 7.9 weeks gestation). Air pollutant exposures were assigned based on residence during the pregnancy. A second cohort study of 1418 children in Boston, Massachusetts, found that children of mothers who lived closest to a major roadway at the time of delivery had a 0.3 kg/m2 higher BMI, a higher waist circumference, and a 40.7% higher leptin concentration (Fleisch et al. 2017).
Three windows of exposure were studied, pregnancy, birth to 1 year, and 1 to 5 years. No pollutants in any of the windows had significant findings and were within the WHO limits. More research is needed to confirm these trends as not all TRAP pollutants were assessed and all studied pollutants had five or fewer studies conducted.
Others (diabetes, thyroid)
Two (n = 2) studies assessed TRAP exposures in relation to other adverse health outcomes including diabetes and thyroid conditions (Hathout et al. 2002; Howe et al. 2018). One of the studies was a case–control and did not list adjustment for potential confounders and the other a cohort-designed study had a lengthy list of confounders including exposure to environmental tobacco smoke. Five of the six TRAP pollutants have been studied. For one of the pollutants assessed (O3), the average exposures were within WHO limits within all their studies with significant findings. Two pollutants (PM10, PM2.5) showed significance, but did not have average exposures within guidelines. In a case–control study of children (less than 5 years of age) diagnosed with diabetes (n = 61 cases) at a hospital in Loma Linda, CA, researchers found an association with childhood exposure to O3 (OR = 3.25, 95%CI: 1.22–8.67) and PM10 (OR = 3.32, 95%CI: 1.10–10.08). Average exposure for O3 was within WHO limits (Hathout et al. 2002). Within this study, cases were identified as children attending the Pediatric Diabetes Center at Loma Linda University Medical Center and matched controls were selected from age-matched healthy children, residing within 100 square miles of the case, and identified through schools and other social settings. Zip codes and dates of residence from birth to diagnosis were used to obtain geographic- and time-specific air concentrations of NO2, O3, SO2, and PM10.
Two windows of exposure have been studied, pregnancy and 1 to 5 years. Within one window of exposure, one pollutant was identified with average exposures within WHO limits for all its significant studies (window and pollutant): 1 to 5 years (O3). Overall, more research is needed to confirm these trends as not all TRAP pollutants were assessed and all studied pollutants had five or fewer studies conducted.
Discussion
Results of this review are indicative of a broad pattern of potential association between TRAP and a range of adverse health outcomes in young children (< 5 years), many within the WHO limits intended to protect vulnerable populations. Across health outcomes, a few pollutants consistently displayed associations. NO2 was the pollutant most extensively studied and found associated with adverse health outcomes. Additionally, O3 and particulates were also found associated with adverse health outcomes.
Most research on health outcomes among young children was done for respiratory diseases, specifically asthma. With lungs having direct contact with pollutants, there is greater potential for immediate and long-term related health effects involving the lungs, for exposed populations. TRAP exposure was found to be associated with increased lung infections (pneumonia, bronchitis, etc.), and chronic conditions, including asthma and increased use of health care services (Bai et al. 2018; Brauer et al. 2007, 2002; Clark et al. 2010; Deng et al. 2016; Gehring et al. 2013). Although all criteria pollutants were found to be related to adverse respiratory outcomes, NO2, PM10, and PM2.5 exposures had the greatest association. For both ear infections and allergies/eczema, exposure to NO2, PM2.5, and O3 displayed the strongest associations. Across both respiratory and allergies/eczema categories, critical windows for TRAP exposure and adverse health outcomes include pregnancy and birth to 1 year, especially for NO2, O3, PM2.5, and SO2.
The remaining adverse health categories and TRAP exposures have received considerably less research. Overall, based on this review and across the categories of development disorders, cancer, obesity, and other conditions, the critical windows of exposure include pregnancy and birth to 1 year. While few studies to date focus on developmental disorders in young children and TRAP exposures, the most commonly assessed outcomes were autism and mental development. For autism, PM2.5 and NO2 were the most consistently associated pollutants, and for mental development, PM10 and NO2. Among pediatric cancers, though only a few studies conducted, retinoblastomas were most commonly associated with TRAP, with significant relationships with NO2, CO, and PM2.5 exposures. Diabetes, obesity, and thyroid were the least researched so far. Though there are potential associations, there is clearly a need for much greater breadth and depth of studies.
Regulatory air quality guidelines
With the range of health conditions linked to TRAP exposures, and many occurring within WHO air quality guidelines, questions arise as to whether or not the current guidelines afford sufficient protection for young children. In every adverse health outcome category, there was at least one pollutant in which all of their significant study findings had average exposures within WHO limits. O3, followed by NO2 and SO2, were the pollutants with the greatest number of disease categories with significant findings within acceptable guidelines. O3 is the pollutant that across all adverse health outcome categories for which it has been studied (not assessed for obesity or cancer categories), all of the significant study findings had average exposures that were within WHO limits. When assessing exposure windows, O3 and NO2 had 100% of significant studies within WHO limits across all but two health categories. These findings suggest that regulatory air quality guidelines may not afford young children sufficient protection from TRAP exposures, especially for NO2, O3 and SO2.
Implications for health professionals
Our findings have strong implications for health professionals, especially those working in pediatric and obstetric healthcare fields. Even within air quality regulatory guidelines, TRAP potentially exacerbate or promote the development of a variety of adverse health outcomes, affecting young children either directly or in utero. For health professionals working with this population, care should be taken to reduce exposures, especially for those living in communities with heightened pollutant exposures (i.e., near major freight railyard, freeways, industrial pollution sources). Persons living in regions with poor air quality are often not fully aware that daily exposures to pollutants affect the health of their unborn baby and/or young children. Health professionals caring for vulnerable populations with increased pollutant exposures should develop and implement strategies for guiding patients and their families to reduce exposure.
Limitations
There are a couple of important limitations to this systematic review that merit discussion. As the systematic review is based on published studies, there exists the potential for publication bias, only publishing air quality studies that find significant adverse health associations. Additionally, a limited number of studies have been published for a number of adverse health categories identified through this review. Lastly, many of the studies assessing O3 or CO exposures are not based on the 8-h exposure time period, which is the time period set for air quality guidelines, though the 24-h or monthly average exposures may be a good estimate of the 8-h time period. In light of these limitations, caution is warranted in interpreting findings, and additionally, research is needed to further shed light on the adverse effects of air pollutant exposures and exposures that are within air quality regulatory guidelines.
Future research opportunities
This systematic review points to the need for further investigations of TRAP exposures and adverse health outcomes. For many of the health outcomes we reviewed, there was only a handful of TRAP and adverse health outcomes studies in children 0–5 years, a critical time window for future health. Also, because several significant health effects were associated with TRAP exposure during pregnancy, it is imperative that future research investigates mechanism at play during the gestational period that could influence these health outcomes. For example, findings that TRAP can alter and cross the placenta and enter the fetus need to be further explored (e Silva et al. 2008; Maisonet et al. 2004). There is also a need to assess how TRAP exposure may alter DNA, especially during the fetal stage (Kim and Hong 2012; Calderón-Garcidueñas et al. 2008). Furthermore, investigation into the under-researched health outcomes identified in this review may identify new mechanistic avenues and risk. Finally, across all conditions, research is needed for subpopulations of children with potentially greater vulnerabilities (i.e., asthmatics, transplants) to TRAP.
Future studies should more accurately assess exposure in children and pregnant women. The use of personal monitors and more precise data of a person’s movements could provide a more accurate exposure picture. Given the multi-factorial nature of health, and as studies are beginning to identify how lifestyle may influence the relationship between TRAP exposure and adverse health outcomes, future studies should adjust for lifestyle and dietary factors (Gharibvand et al. 2016). This opens the possibility for potential impact mitigation of TRAP exposures through of dietary and lifestyle interventions.
There is a need to evaluate if regulatory guidelines adequately protect young children and pregnant women. In addition, research should explore opportunities to empower vulnerable persons to reduce their risk of adverse health outcomes associated with TRAP exposures. Development of mitigation technology to help community members, especially those living in high air pollutant regions and near major local pollution sources, will enable them to protect themselves from TRAP exposure effects.
Conclusion
Air pollutant exposures place children at increased risk for developing a wide range of adverse health conditions with many yet to be fully explored. More research is needed to elucidate the full health impact of ambient air pollutant exposures for young children. In the meantime, while more research efforts are ongoing, health professionals can take action promoting exposure reduction for vulnerable populations and encouraging resilience among children and their families.
Data availability
Database is available for sharing.
Abbreviations
- ADHD:
-
Attention-deficit hyperactive disorder
- ALL:
-
Acute lymphoblastic leukemia
- BBB:
-
Blood-brain barrier
- BMI:
-
Body mass index
- EPA:
-
Environmental Protection Agency
- TRAP:
-
Traffic-related air pollution
- WHO:
-
World Health Organization
References
Aguilera I, Pedersen M, Garcia-Esteban R et al (2012) Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect 121(3):387–392
Bai L, Su X, Zhao D, Zhang Y, Cheng Q, Zhang H, Wang S, Xie M, Su H (2018) Exposure to traffic-related air pollution and acute bronchitis in children: season and age as modifiers. J Epidemiol Community Health 72(5):426–433
Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B (2013) Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect 121(3):380–386
Braga AL, Saldiva PH, Pereira LA et al (2001) Health effects of air pollution exposure on children and adolescents in São Paulo, Brazil. Pediatr Pulmonol 31(2):106–113
Brauer M, Hoek G, Van Vliet P et al (2002) Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 166(8):1092–1098
Brauer M, Gehring U, Brunekreef B et al (2006) Traffic-related air pollution and otitis media. Environ Health Perspect 114(9):1414–1418
Brauer M, Hoek G, Smit HA et al (2007) Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 29(5):879–888
Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C et al (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol 36(2):289–310
Chiu Y-HM, Hsu H-HL, Wilson A et al (2017) Prenatal particulate air pollution exposure and body composition in urban preschool children: examining sensitive windows and sex-specific associations. Environ Res 158:798–805
Clark NA, Demers PA, Karr CJ et al (2010) Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect 118(2):284–290
Deng Q, Lu C, Li Y, Sundell J, Norbäck D (2016) Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res 150:119–127
e Silva IRR, Lichtenfels AJF, Pereira LAA, Saldiva PH (2008) Effects of ambient levels of air pollution generated by traffic on birth and placental weights in mice. Fertil Steril 90(5):1921–1924
EPA (2016) Environmental protection agency: national ambient air quality standards for criteria air pollutants. https://www.epa.gov/criteria-air-pollutants/naaqs-table
Feychting M, Svensson D, Ahlbom A (1998) Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health 24(1):8–11
Fleisch AF, Luttmann-Gibson H, Perng W et al (2017) Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes 12(1):48–57
Fox SE, Levitt P, Nelson CA III (2010) How the timing and quality of early experiences influence the development of brain architecture. Child Dev 81(1):28–40
Gehring U, Cyrys J, Sedlmeir G et al (2002) Traffic-related air pollution and respiratory health during the first 2 yrs of life. Eur Respir J 19(4):690–698
Gehring U, Gruzieva O, Agius Raymond M et al (2013) Air pollution exposure and lung function in children: the ESCAPE Project. Environ Health Perspect 121(11–12):1357–1364
Gehring U, Wijga AH, Hoek G et al (2015) Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 3(12):933–942
Gharibvand L, Ghamsary M, Beeson L, Knutsen R, Soret S, Knutsen S (2016) Does diet modify the association between lung cancer and ambient particulate air pollution? Eur Res J 48(suppl 60)
Ghosh JKC, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B (2013) Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol 178(8):1233–1239
Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D (2004) Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 15(1):36–45
Gruzieva O, Bergström A, Hulchiy O et al (2013) Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology 24(1):54–61
Guxens M, Aguilera I, Ballester F et al (2011) Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect 120(1):144–149
Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW (2002) Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes 3(4):184–188
Heck JE, Wu J, Lombardi C et al (2013) Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect 121(11–12):1385–1391
Hertz-Picciotto I, Baker RJ, Yap P-S et al (2007) Early childhood lower respiratory illness and air pollution. Environ Health Perspect 115(10):1510
Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, Blagev DP, Korgenski EK, Torosyan N, Hansen GI, Kartchner D, Pope CA 3rd (2018) Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med 198(6):759–766
Howe CG, Eckel SP, Habre R et al (2018) Association of prenatal exposure to ambient and traffic-related air pollution with newborn thyroid function: findings from the Children’s Health Study. JAMA Netw Open 1(5):e182172–e182172
Huang C, Wen H, Chen P, Chiang T, Lin S, Guo Y (2015) Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol 173(4):981–988
Jedrychowski WA, Perera FP, Maugeri U et al (2010) Effect of prenatal exposure to fine particulate matter on ventilatory lung function of preschool children of non-smoking mothers. Paediatr Perinat Epidemiol 24(5):492–501
Jedrychowski WA, Perera FP, Spengler JD et al (2013) Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int J Hyg Environ Health 216(4):395–401
Karr C, Lumley T, Shepherd K et al (2005) A case–crossover study of wintertime ambient air pollution and infant bronchiolitis. Environ Health Perspect 114(2):277–281
Karr CJ, Rudra CB, Miller KA et al (2009) Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res 109(3):321–327
Kennedy CM, Pennington AF, Darrow LA et al (2018) Associations of mobile source air pollution during the first year of life with childhood pneumonia, bronchiolitis, and otitis media. Environ Epidemiol 2(1):e007
Kerin T, Volk H, Li W et al (2018) Association between air pollution exposure, cognitive and adaptive function, and ASD severity among children with autism spectrum disorder. J Autism Dev Disord 48(1):137–150
Kim B-J, Hong S-J (2012) Ambient air pollution and allergic diseases in children. Korean J Pediatr 55(6):185–192
Kim E, Park H, Hong Y-C et al (2014) Prenatal exposure to PM10 and NO2 and children’s neurodevelopment from birth to 24 months of age: Mothers and Children’s Environmental Health (MOCEH) study. Sci Total Environ 481:439–445
Kim E, Park H, Park EA et al (2016) Particulate matter and early childhood body weight. Environ Int 94:591–599
Kim Y-M, Kim J, Han Y, Jeon B-H, Cheong H-K, Ahn K (2017) Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS ONE 12(4):e0175229
Kousha T, Castner J (2016) The air quality health index and emergency department visits for Otitis Media. J Nurs Scholarsh 48(2):163–171
Lavigne É, Bélair M-A, Do MT et al (2017) Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int 100:139–147
Lin C-C, Yang S-K, Lin K-C et al (2014) Multilevel analysis of air pollution and early childhood neurobehavioral development. Int J Environ Res Public Health 11(7):6827–6841
Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q (2017) Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci 85(2):85–95
MacIntyre EA, Karr CJ, Koehoorn M, Demers PA, Tamburic L, Lencar C, Brauer M (2011) Residential air pollution and otitis media during the first two years of life. Epidemiology 22(1):81–89
MacIntyre EA, Gehring U, Mölter A et al (2013) Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect 122(1):107–113
Maisonet M, Correa A, Misra D, Jaakkola JJ (2004) A review of the literature on the effects of ambient air pollution on fetal growth. Environ Res 95(1):106–115
Mölter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, de Jongste J, de Vocht F, Fuertes E, Gehring U, Gruzieva O, Heinrich J, Hoek G, Hoffmann B, Klümper C, Korek M, Kuhlbusch TA, Lindley S, Postma D, Tischer C, Wijga A, Pershagen G, Agius R (2015) A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J 45(3):610–624
Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD, Zabaleta C, Sunyer J (2015) Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 70(1):64–73
Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN (2010) Outdoor particulate matter and childhood asthma admissions in Athens, Greece: a time-series study. Environ Health 9(1):45
Nordling E, Berglind N, Melén E, Emenius G, Hallberg J, Nyberg F, Pershagen G, Svartengren M, Wickman M, Bellander T (2008) Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology 19(3):401–408
Raz R, Roberts AL, Lyall K et al (2014) Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case–control analysis within the Nurses’ Health Study II cohort. Environ Health Perspect 123(3):264–270
Rodríguez-Villamizar L, Rojas-Roa N, Blanco-Becerra L, Herrera-Galindo V, Fernández-Niño J (2018) Short-term effects of air pollution on respiratory and circulatory morbidity in Colombia 2011–2014: a multi-city, time-series analysis. Int J Environ Res Public Health 15(8):1610
Salvi S (2007) Health effects of ambient air pollution in children. Paediatr Respir Rev 8(4):275–280
Sbihi H, Tamburic L, Koehoorn M, Brauer M (2016) Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J 47(4):1062–1071
Sentís A, Sunyer J, Dalmau-Bueno A et al (2017) Prenatal and postnatal exposure to NO2 and child attentional function at 4–5 years of age. Environ Int 106:170–177
Song J, Lu M, Zheng L et al (2018) Acute effects of ambient air pollution on outpatient children with respiratory diseases in Shijiazhuang, China. BMC Pulm Med 18(1):150
Šrám RJ, Binková B, Dejmek J, Bobak M (2005) Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 113(4):375
Stern G, Latzin P, Röösli M et al (2013) A prospective study of the impact of air pollution on respiratory symptoms and infections in infants. Am J Respir Crit Care Med 187(12):1341–1348
Vadillo-Ortega F, Osornio-Vargas A, Buxton MA et al (2014) Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses 82(2):219–224
Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R (2013) Traffic-related air pollution, particulate matter, and autism. JAMA Psychiat 70(1):71–77
WHO (2005) WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. http://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf?sequence=1. Accessed Mar 2019
Yang Q, Chen Y, Krewski D, Shi Y, Burnett RT, Mcgrail KM (2004) Association between particulate air pollution and first hospital admission for childhood respiratory illness in Vancouver, Canada. Arch Environl Health Int J 59(1):14–21
Zemek R, Szyszkowicz M, Rowe BH (2010) Air pollution and emergency department visits for otitis media: a case-crossover study in Edmonton, Canada. Environ Health Perspect 118(11):1631
Zhu L, Ge X, Chen Y et al (2017) Short-term effects of ambient air pollution and childhood lower respiratory diseases. Sci Rep 7(1):4414
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
Authors give consent for publication
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Table of contents summary
Ambient air pollutants are linked with a wide range of adverse health outcomes in early childhood and occurring within WHO/EPA air quality regulatory guidelines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spencer-Hwang, R., Hwang, J., Sinclair, R. et al. Adverse health outcomes in early childhood (birth to 5 years) and ambient air pollutant exposures: a systematic review. Air Qual Atmos Health 16, 913–944 (2023). https://doi.org/10.1007/s11869-023-01308-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01308-1