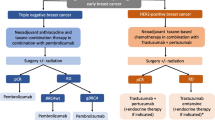
Opinion statement
Human epidermal growth factor receptor 2-positive (HER2+) breast cancers have been historically considered an aggressive entity with high rates of recurrence and poor survival. However, during the last 20 years, there has been a dramatic change in prognosis due to the incorporation of different anti-HER2 therapies into the neo/adjuvant chemotherapy backbone. Neoadjuvant dual blockade with trastuzumab and pertuzumab has become the standard of care for women with stage II and III HER2+ breast cancer. Trastuzumab emtansine (T-DM1) has been shown to improve outcomes if pathological complete response (pCR) is not achieved, and adjuvant extended therapy with neratinib has increased disease-free survival (DFS) and may have an impact in central nervous system (CNS) recurrences. However, these agents are both toxic for individual patients and costly for the overall healthcare system, and there are still patients that experience recurrence despite therapy improvements. At the same time, it has been shown that some patients with early-stage HER2+ breast cancer can be effectively treated with less intensive systemic therapy, using only taxane and trastuzumab, or that the chemotherapy backbone can be omitted completely. The current challenge is to properly identify which patients can receive a de-intensified regimen and which need new intensification strategies. Tumor size, nodal status, and pCR achievement after neoadjuvant treatment are well-known risk factors that can aid in making clinical decisions, but they do not accurately predict all patient outcomes. Various biomarkers have been proposed to better characterize the clinical and biological heterogeneity of HER2+ breast cancer. Immune infiltration, intrinsic subtype, intratumoral heterogeneity, and dynamic changes during treatment have been described as important prognostic and/or predictive features. The integration of all these factors will be key in the proper identification of the true risk, and individualized treatment strategy, for each patient.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Slamon D, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. https://doi.org/10.1126/science.3798106.
Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28:963–8.
Sørlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. https://doi.org/10.1073/pnas.0932692100.
Slamon D, et al. Adjuvant trastuzumab in HER2-positive breast cancer. Protocol N Engl J Med. 2011. https://doi.org/10.1056/NEJMoa0910383.
Perez EA, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2 - Positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014. https://doi.org/10.1200/JCO.2014.55.5730.
Bradley, R. et al. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021. https://doi.org/10.1016/S1470-2045(21)00288-6. Meta-analysis showing a reduction in the risk of recurrence and mortality in patients with HER2+ early breast cancer treated with chemotherapy with trastuzumab versus chemotherapy alone.
Von Minckwitz G, et al. APHINITY trial (BIG 4–11): A randomized comparison of chemotherapy (C) plus trastuzumab (T) plus placebo (Pla) versus chemotherapy plus trastuzumab (T) plus pertuzumab (P) as adjuvant therapy in patients (pts) with HER2-positive early breast cancer (EBC). J Clin Oncol. 2017. https://doi.org/10.1200/jco.2017.35.18_suppl.lba500.
von Minckwitz G, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019. https://doi.org/10.1056/nejmoa1814017. Randomized phase III trial in patients with HER2+ early breast cancer and residual invasive disease after completion of neoadjuvant therapy. Significant reduction in the risk of recurrence with adjuvant T-DM1 compared to adjuvant trastuzumab.
Chan A, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016. https://doi.org/10.1016/S1470-2045(15)00551-3.
Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012. https://doi.org/10.1093/annonc/mds324.
Cortazar P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014. https://doi.org/10.1016/S0140-6736(13)62422-8.
Huober J, et al. Factors predicting relapse in early breast cancer patients with a pathological complete response after neoadjuvant therapy: pooled analysis based on the GBG database. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz097.
Loibl S, et al. VP6–2022: adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 84 years’ follow-up. Ann Oncol. 2022;33:986–7.
Chan A, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021. https://doi.org/10.1016/j.clbc.2020.09.014. Final long-term follow-up results from the randomized phase III trial of adjuvant neratinib versus placebo in patients with HER2+ early breast cancer who have completed adjuvant trastuzumab, including a sub-analysis in HR+ patients.
Lin NU, Winer EP. Brain metastases: The HER2 paradigm. Clin Cancer Res. 2007. https://doi.org/10.1158/1078-0432.CCR-06-2478.
Ferraro E, et al. Incidence of brain metastases in patients with early HER2-positive breast cancer receiving neoadjuvant chemotherapy with trastuzumab and pertuzumab. NPJ Breast Cancer. 2022. https://doi.org/10.1038/s41523-022-00380-7.
Barcenas CH, et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020. https://doi.org/10.1016/j.annonc.2020.05.012.
Loi S, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013. https://doi.org/10.1200/JCO.2011.41.0902.
Park SE, et al. Clinical implication of tumor mutational burden in patients with HER2-positive refractory metastatic breast cancer. Oncoimmunology. 2018. https://doi.org/10.1080/2162402X.2018.1466768.
Bianchini G, et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol. 2015. https://doi.org/10.1093/annonc/mdv395.
Park SG, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010. https://doi.org/10.1016/j.ccr.2010.06.014.
Müller P, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.aac4925.
Huober J, et al. Atezolizumab with neoadjuvant anti–human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol. 2022;40:2946–56.
Loibl S, et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neoadjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J Clin Oncol. 2021. https://doi.org/10.1200/jco.2021.39.15_suppl.506.
Van Der Voort A, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2 -positive breast cancer: a secondary analysis of the TRAIN-2 randomized, phase 3 trial. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.1371. Follow-up analysis of the randomized phase III TRAIN-2 using a regimen with or without anthracyclines with dual HER2 blockade for stage II and III HER2+ breast cancer. Similar recurrence and survival secondary endpoints regardless of anthracycline receipt.
Schneeweiss A, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013. https://doi.org/10.1093/annonc/mdt182.
Schneeweiss A, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive ea. Eur J Cancer. 2018. https://doi.org/10.1016/j.ejca.2017.10.021.
Tarantino P, Tolaney SM, Harbeck N, Cortes J, Curigliano G. Anthracyclines for human epidermal growth factor receptor 2-positive breast cancer: are we ready to let them go? J Clin Oncol. 2021. https://doi.org/10.1200/JCO.21.01059.
Gradishar WJ et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. Natl Compr Canc Netw. 2022;20:691-722. https://doi.org/10.6004/jnccn.2022.0030.
Tolaney SM, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015. https://doi.org/10.1056/nejmoa1406281.
Tolaney SM et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. https://doi.org/10.1016/S1470-2045(23)00051-7. Update of the phase II single-arm trial of adjuvant trastuzumab and paclitaxel in small, node-negative, HER2+ breast cancers. Excellent long-term outcomes in this low-risk population treated with a de-escalated adjuvant regimen.
Tarantino P, et al. PD18–01; Adjuvant trastuzumab emtansine versus paclitaxel plus trastuzumab for stage I HER2+ breast cancer: 5-year results and correlative analyses from ATEMPT (TBCRC033). San Antonio Breast Cancer Symposium, December 6–10, 2022. San Antonio, Texas.
Weiss A, et al. Abstract P2–13-02: Pathologic nodal staging and systemic therapy among patients with cT1-2N0 HER2+ breast cancer: a prospective single institution cohort analysis. Cancer Res. 2022. https://doi.org/10.1158/1538-7445.sabcs21-p2-13-02.
Martinez Saez O, et al. 108P Pathologic nodal positivity in patients with cT1-2 cN0 HER2+ breast cancer treated with upfront surgery or neoadjuvant anti-HER2-based therapy. Ann Oncol. 2022;33:S174.
Waks AG, et al. A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer. NPJ Breast Cancer. 2022;8. https://doi.org/10.1038/s41523-022-00429-7.
Nitz UA, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR- phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly pacl. Ann Oncol. 2017. https://doi.org/10.1093/annonc/mdx494.
Harbeck N, et al. De-escalated neoadjuvant pertuzumab + trastuzumab with or without paclitaxel weekly in HR-/HER2+ early breast cancer: ADAPT-HR-/HER2+ biomarker and survival results. J Clin Oncol. 2021. https://doi.org/10.1200/jco.2021.39.15_suppl.503.
Nitz U, Gluz O, Graeser M, Christgen M, Kuemmel S, Grischke E-M, et al. De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR–): survival outcomes from a multicentre, open-label, randomised, phase 2 tr. Lancet Oncol. 2022;23:625–35.
Pérez-García JM, et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021. https://doi.org/10.1016/S1470-2045(21)00122-4. Non-comparative, phase II trial assessing early metabolic responses to neoadjuvant trastuzumab and pertuzumab using 18F-FDG-PET and the possibility of chemotherapy omission using a pCR-adapted strategy in patients with HER2+, stage I-IIIA breast cancer.
Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. https://doi.org/10.1056/nejmoa052306.
Earl HM, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019. https://doi.org/10.1016/S0140-6736(19)30650-6.
Pivot X, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019. https://doi.org/10.1016/S0140-6736(19)30653-1.
Mavroudis D, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015. https://doi.org/10.1093/annonc/mdv213.
Joensuu H, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: The SOLD randomized clinical trial. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.1380.
Conte P, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study. Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdy414.
Earl HM, et al. LBA11 Individual patient data meta-analysis of 5 non-inferiority RCTs of reduced duration single agent adjuvant trastuzumab in the treatment of HER2 positive early breast cancer. Ann Oncol. 2021. https://doi.org/10.1016/j.annonc.2021.08.2083.
Prat A, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014. https://doi.org/10.1093/jnci/dju152.
Prat A, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020. https://doi.org/10.1093/jnci/djz042.
Schettini F, et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2020. https://doi.org/10.1016/j.ctrv.2020.101965. Meta-analysis assessing the association of HER2-enriched subtype with pCR following HER2-directed neoadjuvant treatments with or without chemotherapy.
Nuciforo P, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdx647.
Denkert C, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018. https://doi.org/10.1016/S1470-2045(17)30904-X.
Solinas C, et al. Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: a meta-analysis of randomized controlled trials. Cancer Treat Rev. 2017. https://doi.org/10.1016/j.ctrv.2017.04.005.
Perez EA, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the n9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016. https://doi.org/10.1001/jamaoncol.2015.3239.
Locy H, et al. Assessing tumor-infiltrating lymphocytes in breast cancer: a proposal for combining immunohistochemistry and gene expression analysis to refine scoring. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.794175.
Chumsri S, et al. Association between adaptive immune signature and outcome in HER2-positive breast cancer treated with trastuzumab and lapatinib in the NCCTG-N9831 (Alliance) and NeoALTTO trials. J Clin Oncol. 2018. https://doi.org/10.1200/jco.2018.36.15_suppl.577.
Fernandez-Martinez A, et al. Survival, pathologic response, and genomics in CALGB 40601 (Alliance), a neoadjuvant Phase III trial of paclitaxel-trastuzumab with or without lapatinib in HER2-positive breast cancer. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.20.01276.
Krop IE, et al. Genomic correlates of response to adjuvant trastuzumab (H) and pertuzumab (P) in HER2+ breast cancer (BC): Biomarker analysis of the APHINITY trial. J Clin Oncol. 2019. https://doi.org/10.1200/jco.2019.37.15_suppl.1012.
Iglesia MD, et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014. https://doi.org/10.1158/1078-0432.CCR-13-3368.
Fan C, et al. Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures. BMC Med Genomics. 2011. https://doi.org/10.1186/1755-8794-4-3.
Fernandez-Martinez A, et al. 120MO Prognostic value of immune gene-expression signatures (iGES) vs tumor-infiltrating lymphocytes (TILs) in early-stage HER2+ breast cancer: a combined analysis of CALGB 40601 (C40601) and PAMELA trials. Ann Oncol. 2021. https://doi.org/10.1016/j.annonc.2021.08.401.
Prat A, et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. eBioMedicine. 2022. https://doi.org/10.1016/j.ebiom.2021.103801. Development and validation of the HER2DX predictive and prognostic tests, based on a 27-gene expression plus clinical feature-based classifier, in patients with HER2+ early breast cancer.
Carey LA, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016. https://doi.org/10.1200/JCO.2015.62.1268.
Fumagalli D, et al. RNA sequencing to predict response to neoadjuvant anti-HER2 therapy a secondary analysis of the NeoALTTO randomized clinical trial. JAMA Oncol. 2017. https://doi.org/10.1001/jamaoncol.2016.3824.
Triulzi T, et al. Whole-transcriptome analysis links trastuzumab sensitivity of breast tumors to both HER2 dependence and immune cell infiltration. Oncotarget. 2015. https://doi.org/10.18632/oncotarget.4405.
Pogue-Geile KL, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst. 2013. https://doi.org/10.1093/jnci/djt321.
Denkert C, et al. Biomarker data from KATHERINE: a phase III study of adjuvant trastuzumab emtansine (T-DM1) versus trastuzumab (H) in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer. J Clin Oncol. 2020. https://doi.org/10.1200/jco.2020.38.15_suppl.502. Exploratory analyses of predictive biomarkers in residual disease tumor samples of patients receiving adjuvant T-DM1 or trastuzumab in the phase III KATHERINE trial.
Wilson TR, et al. Development of a robust RNA-based classifier to accurately determine ER, PR, and HER2 status in breast cancer clinical samples. Breast Cancer Res Treat. 2014. https://doi.org/10.1007/s10549-014-3163-8.
Berns K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007. https://doi.org/10.1016/j.ccr.2007.08.030.
Goel S, Krop IE. PIK3CA mutations in HER2-positive breast cancer: an ongoing conundrum. Ann Oncol. 2016. https://doi.org/10.1093/annonc/mdw246.
Seo Y, et al. PIK3CA mutations and neoadjuvant therapy outcome in patients with human epidermal growth factor receptor 2-positive breast cancer: a sequential analysis. J Breast Cancer. 2018. https://doi.org/10.4048/jbc.2018.21.e48.
Majewski IJ, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015. https://doi.org/10.1200/JCO.2014.55.2158.
Loibl S, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (HER2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014. https://doi.org/10.1200/JCO.2014.55.7876.
Chia SKL, et al. PIK3CA alterations and benefit with neratinib: analysis from the randomized, double-blind, placebo-controlled, phase III ExteNET trial. Breast Cancer Res. 2019. https://doi.org/10.1186/s13058-019-1115-2.
Nagata Y, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004. https://doi.org/10.1016/j.ccr.2004.06.022.
Hurvitz SA, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2–positive breast cancer: three-year outcomes from the phase III KristinE study. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.19.00882.
Metzger Filho O, et al. HER2 heterogeneity as a predictor of response to neoadjuvant T-DM1 plus pertuzumab: Results from a prospective clinical trial. J Clin Oncol. 2019. https://doi.org/10.1200/jco.2019.37.15_suppl.502.
Prat A, et al. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: a retrospective study with an external evaluation. Lancet Oncol. 2020. https://doi.org/10.1016/S1470-2045(20)30450-2.
Veeraraghavan J, et al. A multiparameter classifier to predict response to lapatinib plus trastuzumab (LT) without chemotherapy in HER2+ breast cancer (BC). J Clin Oncol. 2020. https://doi.org/10.1200/jco.2020.38.15_suppl.1011.
Pernas S, et al. Early on-treatment vs pre-treatment tumour transcriptomes as predictors of response to neoadjuvant therapy for HER2-positive inflammatory breast cancer. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz239.031.
Guarneri V, et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz055.
Hurvitz SA, et al. Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07). Nat Commun. 2020. https://doi.org/10.1038/s41467-020-19494-2.
Chic N, et al. Tumor cellularity and infiltrating lymphocytes as a survival surrogate in HER2-positive breast cancer. J Natl Cancer Inst. 2022. https://doi.org/10.1093/jnci/djab057.
Brasó-Maristany F, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020. https://doi.org/10.1038/s41467-019-14111-3.
Hatschek T, et al. Neoadjuvant trastuzumab, pertuzumab, and docetaxel vs trastuzumab emtansine in patients with ERBB2-positive breast cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.1932.
Connolly RM, et al. Updated results of TBCRC026: phase II Trial correlating standardized uptake value with pathological complete response to pertuzumab and trastuzumab in breast cancer. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.21.00280.
Rothé F, et al. Precision medicine and imaging circulating tumor DNA in HER2-amplified breast cancer: a translational research substudy of the NeoALTTO Phase III Trial. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.CCR-18-2521.
Acknowledgements
Olga Martínez-Sáez is a SEOM Visiting Fellow 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Olga Martínez-Sáez reports travel expenses and consulting fees from Roche and Reveal Genomics and speaker fees from Eisai and Novartis. Adrienne G. Waks has served in an advisory board for AstraZeneca and she declares institutional research support by Macrogenics, Genentech, Merck and Verastem Oncology.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez-Sáez, O., Waks, A.G. Individualizing Curative-Intent Therapy in HER2-Positive Early-Stage Breast Cancer. Curr. Treat. Options in Oncol. 24, 479–495 (2023). https://doi.org/10.1007/s11864-023-01070-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01070-7