Abstract
Background
Sestrin-2 (SESN2) is a antioxidant protein that can be activated by a number of conditions, including DNA damage and hypoxia.
Aims
Our objective was to evaluate maternal serum SESN2 levels in patients with intrauterine growth restriction (IUGR) and its association with adverse perinatal outcomes.
Methods
This prospective study included a total of 87 pregnant women admitted to our tertiary care center between 2018 August and 2019 July. The study group consisted of a total of 44 patients who had been diagnosed with IUGR. Forty-three low-risk and gestational age-matched pregnant women were taken as control group. Demographic data, maternal serum SESN2 levels, and maternal-neonatal outcomes were evaluated. SESN2 levels were analyzed by the enzyme-linked immunosorbent assay (ELISA) method and compared between groups.
Results
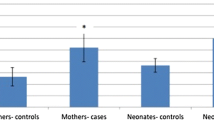
Maternal serum SESN2 levels were significantly higher in the IUGR group compared to control group (22.38 ng/ml vs. 13.0 ng/ml, p < 0.001). In correlation analysis, a negative significant correlation was found between SESN2 levels and gestational week at delivery (r = − 0.387, p < 0.001). The ideal cut-off value for detecting IUGR was 9.5 ng/ml, and the area under the curve was 0.719 (95%CI: 0.610–0.827). Birth interval, gestational week at birth, birth weight, and 1–5-min Apgar scores were lower in the IUGR group (p < 0.001).
Conclusions
Maternal serum SESN2 levels are elevated in IUGR and are associated with adverse neonatal outcome. Considering that SESN2 is involved in pathogenesis, it can be used as a new marker for the evaluation of IUGR.

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society forMaternal-FetalMedicin (2019) ACOG Practice Bulletin No. 204: fetal growth restriction. Obstet Gynecol 133:e97–e109
Burton GJ, Redman CW, Roberts JM, Moffett A (2019) Pre-eclampsia: pathophysiology and clinical implications. BMJ 366:l2381
Chen HL, Yang YP, Hu XL et al (1991) Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol 139:327–335
Baack ML, Wang C, Hu S et al (2014) Hyperglycemia induces embryopathy, even in the absence of systemic maternal diabetes: an in vivo test of the fuel mediated teratogenesis hypothesis. Reprod Toxicol 46:129–136
Kameda T, Matsuzaki N, Sawai K et al (1990) Production of interleukin-6 by normal human trophoblast. Placenta 11:205–213
Benyo DF, Miles TM, Conrad KP (1997) Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab 82:1582–1588
Buckbinder L, Talbott R, Seizinger BR, Kley N (1994) Gene regulation by temperature-sensitive p53 mutants: identification of p53 response genes. Proc Natl Acad Sci U S A 91:10640–10644
Velasco-Miguel S, Buckbinder L, Jean P et al (1999) PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 18:127–137
Budanov AV, Shoshani T, Faerman A et al (2002) Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21:6017–6031
Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134:451–460
Peng M, Yin N, Li MO (2014) Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell 159:122–133
Chantranupong L, Wolfson RL, Orozco JM et al (2014) The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9:1–8
Budanov AV, Lee JH, Karin M (2010) Stressin’ Sestrins take an aging fight. EMBO Mol Med 2:388–400
Kim SJ, Kim KM, Yang JH et al (2017) Sestrin2 protects against acetaminophen-induced liver injury. Chem Biol Interact 269:50–58
Shin BY, Jin SH, Cho IJ, Ki SH (2012) Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med 53:834–841
Bae SH, Sung SH, Oh SY et al (2013) Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab 17:73–84
Jegal KH, Ko HL, Park SM et al (2016) Eupatilin induces Sestrin2-dependent autophagy to prevent oxidative stress. Apoptosis 21:642–656
Saker M, Soulimane Mokhtari N, Merzouk SA et al (2008) Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gynecol Reprod Biol 141:95–99
Gordijn SJ, Beune IM, Thilaganathan B et al (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48:333–339
Guvendag Guven ES, Karcaaltincaba D, Kandemir O et al (2013) Cord blood oxidative stress markers correlate with umbilical artery pulsatility in fetal growth restriction. J Matern Fetal Neonatal Med 26:576–580
Granger JP, LaMarca BB, Cockrell K et al (2006) Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122:383–392
Pardi G, Marconi AM, Cetin I (2002) Placental-fetal interrelationship in IUGR fetuses—a review. Placenta 23(Suppl A):S136–S141
Karowicz-Bilińska A, Suzin J, Sieroszewski P (2002) Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med Sci Monit 8:CR211–CR216
Saeedi V, Nourbakhsh M, Haghighi L et al (2021) Sestrin2 and Beclin1 levels in polycystic ovary syndrome. J Clin Lab Anal 35:e23957
Tayyar AT, Tayyar A, Kozali S et al (2019) Maternal serum sestrin 2 levels in preeclampsia and their relationship with the severity of the disease. Hypertens Pregnancy 38:13–19
Asadi N, Roozmeh S, Vafaei H et al (2022) Effectiveness of pentoxifylline in severe early-onset fetal growth restriction: a randomized double-blinded clinical trial. Taiwan J Obstet Gynecol 61:612–619
Yung HW, Alnæs-Katjavivi P, Jones CJ et al (2016) Placental endoplasmic reticulum stress in gestational diabetes: the potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia 59:2240–2250
Lee S, Shin J, Hong Y et al (2020) Sestrin2 alleviates palmitate-induced endoplasmic reticulum stress, apoptosis, and defective invasion of human trophoblast cells. Am J Reprod Immunol 83:e13222
Mitchell BM, Cook LG, Danchuk S, Puschett JB (2007) Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. Am J Hypertens 20:1297–1304
Potdar N, Singh R, Mistry V et al (2009) First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG 116:637–642
Ye J, Wang M, Xu Y et al (2017) Sestrins increase in patients with coronary artery disease and associate with the severity of coronary stenosis. Clin Chim Acta 472:51–57
Krause-Hauch M, Fedorova J, Zoungrana LI et al (2022) Targeting on Nrf2/Sesn2 signaling to rescue cardiac dysfunction during high-fat diet-induced obesity. Cells 11
Author information
Authors and Affiliations
Contributions
MO Agaoglu: project development, data collection, manuscript writing. Z Agaoglu: data collection, data analysis. KY Yucel: data collection. FH Ozturk: data analysis. T Caglar: project development, manuscript writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. All procedures were approved by the Ethics committee of the Zekai Tahir Burak Research Hospital, Ankara, Turkey (Approval No.: 82/2019).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agaoglu, M.O., Agaoglu, Z., Yucel, K.Y. et al. Evaluation of maternal serum sestrin-2 levels in intrauterine growth restriction. Ir J Med Sci 192, 2259–2264 (2023). https://doi.org/10.1007/s11845-023-03329-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03329-2