Abstract
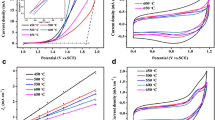
The oxygen evolution kinetics of industrial copper electrodeposition is slow, resulting in low electrocatalytic activity and high energy consumption. In this work, a quaternary composite of carbon coated active particles containing Mn, Co and Ce were prepared (Mn-Co3O4/CeO2@C), and Ti/Sb-SnO2/PbO2 electrode doped with these active particles was prepared by co-electrodeposition. The microstructure and chemical composition of the electrode was characterized by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and X-ray diffractometry (XRD). Linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS) and Tafel polarization curve (Tafel) were used to study the electrochemical properties of anode materials. The results showed that the doping of Mn-Co3O4/CeO2@C active particles promoted the crystal transition of PbO2, decreased the average grain size, and the doping of Ce increases the average valence state of Co. The modified titanium electrode showed excellent catalytic activity of the oxygen evolution reaction (OER) characteristics. The overpotential of the doped Ti/Sb-SnO2/PbO2 anode was only 453 mV when the current density was 20 mA cm−2 in 0.5 M H2SO4 solution, which is 508 mV lower than that of the undoped Ti/Sb-SnO2/PbO2 anode. In simulated copper electro-deposition experiments, the cell voltage was reduced by about 400 mV, compared to the undoped Ti/Sb-SnO2/PbO2 electrode.
Similar content being viewed by others
References
L. Ding, J. Chen, T. Wang, J. Zhao, C. Chen and Y. Niu, Miner. Eng., 135, 21 (2019).
M. Stelter and H. Bombach, Adv. Eng. Mater., 6, 558 (2004).
Y. Liu, W. Zhu, Z. Chen, Q. Yu, Q. Hu, Z. Zheng, L. Gui and Y. Song, Int. J. Hydrogen Energy, 46, 6380 (2021).
V. Krstić and B. Pešovski, Hydrometallurgy, 185, 71 (2019).
L. C. Espinoza, P. Sepúlveda, A. García, D. M. d. Godoi and R. Salazar, Chemosphere, 251, 126674 (2020).
H. W. Lim, D. K. Cho, J. H. Park, S. G. Ji, Y. J. Ahn, J. Y. Kim and C. W. Lee, ACS Catal., 11, 12423 (2021).
A. Touni, O. A. Grammenos, A. Banti, D. Karfaridis, C. Prochaska, D. Lambropoulou, E. Pavlidou and S. Sotiropoulos, Electrochim. Acta, 390, 138866 (2021).
S. Pan, H. Li, D. Liu, R. Huang, X. Pan, D. Ren, J. Li, M. Shakouri, Q. Zhang, M. Wang, C. Wei, L. Mai, B. Zhang, Z. Wang, M. Graetzel and X. Zhang, Nat. Commun., 13, 2294 (2022).
C. Pasquini, I. Zaharieva, D. González-Flores, P. Chernev, M. R. Mohammadi, L. Guidoni, R. D. L. Smith and H. Dau, J. Am. Chem. Soc., 141, 2938 (2019).
X. Yang, H. Li, A. Lu, S. Min, Z. Idriss, M. N. Hedhili, K. Huang, H. Idriss and L. Li, Nano Energy, 25, 42 (2016).
A. Li, S. Kong, C. Guo, H. Ooka, K. Adachi, D. Hashizume, Q. Jiang, H. Han, J. Xiao and R. Nakamura, Nat. Catal., 5, 109 (2022).
B. Chen, W. Yan, Y. He, H. Huang, H. Leng, Z. Guo and J. Liu, J. Electrochem. Soc., 166, 119 (2019).
X. Wang, L. Wang, D. Wu, D. Yuan, H. Ge and X. Wu, Sci. Total Environ., 855, 158880 (2023).
T. Lwai, M. Murakami, S. Takai, T. Yabutsuka and T. Yao, J. Alloy Campd., 780, 85 (2019).
K. Irikura, N. Bocchi, R. C. Rocha-Filho, S. R. Biaggio, J. Iniesta and V. Montiel, J. Environ. Manage, 183, 306 (2016).
S. Chen, B. Chen, S. Wang, W. Yan, Y. He, Z. Guo and R. Xu, J. Alloy. Compd., 815, 152551 (2020).
B. Yu, R. Xu, B. Chen, X. Wang and S. He, Int. J. Hydrogen Energy, 48, 11131 (2023).
S. He, R. Xu, L. Sun, Y. Fan, Z. Zhao, H. Liu and H. Lv, Hydrometallurgy, 194, 105357 (2020).
C. Zhang, J. Liu and B. Chen, Ceram. Int., 44, 19735 (2018).
X. Wang, J. Wang, W. Jiang, C. Chen, B. Yu and R. Xu, Sep. Purif. Technol., 272, 118916 (2021).
J. Wei, J. Wang, X. Wang, W. Jiang, N. Hu, L. Wang, M. Li, R. Xu and L. Yang, Electrochim. Acta, 432, 141221 (2022).
X. Wang, J. Wang, B. Yu, W. Jiang, J. Wei, B. Chen, R. Xu and L. Yang, J. Hazard. Mater., 428, 128212 (2022).
C. Tang, Y. Lu, F. Wang, H. Niu, L. Yu and J. Xue, Electrochim. Acta, 331, 165381 (2020).
Y. Liu, T. Sun, Q. Su, Y. Tang, X. Xu, M. Akram and B. Jiang, J. Colloid Interface Sci., 575, 254 (2020).
W. Alnoush, R. Black and D. Higgins, Chem. Catal., 1, 997 (2021).
Z. Zhao, Y. Long, S. Luo, Y. Luo, M. Chen and J. Ma, J. Energy Chem., 60, 546 (2021).
H. Kim, E. Hwang, H. Park, B. Lee, J. H. Jang, H. Kim, S. H. Ahn and S. Kim, Appl. Catal. B-Environ., 206, 608 (2017).
O. Shmychkova, T. Luk’yanenko, R. Amadelli and A. Velichenko, J. Electroanal. Chem., 706, 86 (2013).
H. Jin, X. Zhang, Y. Yu and X. Chen, Chem. Eng. J., 435, 135167 (2022).
Y. Liu, C. Ma, Q. Zhang, W. Wang, P. Pan, L. Gu, D. Xu, J. Bao and Z. Dai, Adv. Mater., 31, 1900062 (2019).
Z. Wei, X. Kang, S. Xu, X. Zhou, B. Jia and Q. Feng, Chin. J. Chem. Eng., 32, 191 (2021).
T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 5, 13801 (2015).
C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 135, 16977 (2013).
J. Huang, H. Sheng, R. D. Ross, J. Han, X. Wang, B. Song and S. Jin, Nat. Commun., 12, 3036 (2021).
Acknowledgements
The authors are grateful for the financial support from the Shaanxi Science and Technology Department (2022GY-384, 2022JBGS2-07, 2021LLRH-05-21, S2022-YD-QFY-0107), the Open Foundation of Key Laboratory of Synthetic and Natural Functional Molecular Chemistry of Ministry of Education (KLSNFM2020001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yin, Z., He, R., Nie, F. et al. The electrocatalysis of Mn-Co3O4/CeO2@C particles with different Ce content modified Ti/PbO2 anode and its application for copper electrodeposition. Korean J. Chem. Eng. 40, 3059–3067 (2023). https://doi.org/10.1007/s11814-023-1538-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-023-1538-4