Abstract
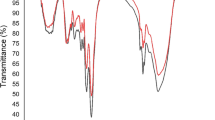
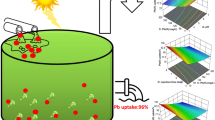
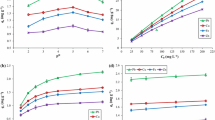
The mycelial growth kinetics, cadmium biosorption capacity and main governing biosorption mechanism of Pleurotus ostreatus (oyster mushroom) have been determined in this study. The fungus mycelium exhibits a sigmoidal (S-shaped) growth curve in which the growth rates for the lag and exponential phases are 0.1 and 0.31 g/L·day, respectively. The grown fungus is subjected to elemental, infra-red and scanning electron microscopy-energy dispersive x-ray spectroscopy analyses, while biosorption data are fitted to established adsorption isotherm models, namely, Langmuir, Freundlich and Dubinin-Radushkevich. It is strongly suggested that the main governing mechanism involved is chemisorption due to good fitting of biosorption data to Langmuir and Dubinin-Radushkevich models with possibility of involvement of both ion exchange and complexation. Data presented in the study are very useful for design of future pilot- or industrial-scale biosorption water purification systems.
Similar content being viewed by others
References
E. Romera, F. Gonzalez, A. Ballester, M. L. Blazquez and J. A. Munoz, Biores. Technol., 98, 3344 (2007).
K. C. Bhainsa and S. F. D’souza, Biores. Technol., 99, 3829 (2007).
Z. Xuan, Y. Tang, X. Li, Y. Liu and F. Luo, Biochem. Eng. J., 31, 160 (2006).
D. P. Mungasavalli, T. Viraraghavan and Y. C. Jin, Colloids Surf. A, 301, 214 (2007).
R. Kumar, N. R. Bishnoi, Garima and K. Bishnoi, Chem. Eng. J., 135, 202 (2008).
M. J. Melgar, J. Alonso and M. A. García, Sci. Total Environ., 385, 12 (2007).
M.G. Peter and U. Wollenberger, in: Frontiers in biosensorics, F.W. Scheller, F. Schubert and J. Fedrowitz Eds., Birkhäuser, Basel (1997).
X. Pan, J. Wang and D. Zhang, Proc. Biochem., 40, 2799 (2005).
X. Pan, J. Wang and D. Zhang, Inter. J. Environ. Poll., 37, 289 (2009).
J.-Z. Wu, P. C. K. Cheung, K.-H. Wong and N.-L. Huang, Food Chem., 81, 389 (2003).
I. Langmuir, J. Am. Chem. Soc., 38, 2221 (1916).
H. Freundlich, Phys. Chem. Soc., 40, 1361 (1906).
M.D. Mashitah, Y. Yus-Azila and S. Bhatia, Biores. Technol., 99, 4742 (2008).
G. Yan and T. Viraraghavan, Water Res., 37, 4486 (2003).
M. M. Dubinin and L. V. Radushkevich, Proc. Acad. Sci. Phys. Chem. Sect. USSR, 55, 331 (1947).
H. Arslanoglu, H. S. Altundogan and F. Tumen, J. Hazard. Mater., 164, 1406 (2009).
J. P. Hobson, J. Phys. Chem., 73, 2720 (1969).
F. Helfferich, Ion exchange, McGraw-Hill, New York (1962).
G. Chen, G. Zeng, L. Tang, C. Du, X. Jiang, G. Huang, H. Liu and G. Shen, Biores. Technol., 99, 7034 (2008).
H. Ginterova and H. Maxianova, Folia Microbiol., 20, 246 (1975).
M. Bhanoori and G. Venkateswerlu, Biochim. Biophys. Acta, 1523, 21 (2000).
B.Y. M. Bueno, M. L. Torem, F. Molina and L. M. S. Mesquita, Miner. Eng., 21, 65 (2008).
G. Olivieri, A. Marzocchella, P. Salatino, P. Giardina, G. Cennamo and G. Sannia, Biochem. Eng. J., 31, 180 (2006).
L. Svecova, M. Spanelova, M. Kubal and E. Guibal, Sep. Purif. Technol., 52, 142 (2006).
Salony, S. Mishra and V. S. Bisaria, Appl. Microbiol. Biotechnol., 71, 646 (2006).
G. Bayramoglu and M.Y. Arica, Chem. Eng. J., 143, 133 (2008).
T. Akar, Z. Kaynak, S. Ulusoy, D. Yuvaci, G. Ozsari and S. T. Akar, J. Hazard. Mater., 163, 1134 (2009).
D. L. Pavia, G. M. Lampman and G. S. Kriz, Introduction to spectroscopy: A guide for students of organic chemistry, Saunders, New York (1996).
M. Fereidouni, A. Daneshi and H. Younesi, J. Hazard. Mater., 168, 1437 (2009).
G. Li, P. Xue, C. Yan and Q. Li, Korean J. Chem. Eng., 27, 1239 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
To be included as footnote: Experimental studies presented in this article were undertaken at Faculty of Civil Engineering, Universiti Teknologi MARA, Malaysia
Rights and permissions
About this article
Cite this article
Tay, C.C., Liew, H.H., Yin, CY. et al. Biosorption of cadmium ions using Pleurotus ostreatus: Growth kinetics, isotherm study and biosorption mechanism. Korean J. Chem. Eng. 28, 825–830 (2011). https://doi.org/10.1007/s11814-010-0435-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0435-9