Abstract
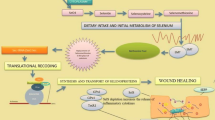
The present study investigated the anti-osteoporosis function and the mechanism of sialoglycoproteins isolated from the eggs of Gadus morhua (Gm-SGP) on ovariectomized (OVX) rats. After 3 months of Gm-SGP treatment, OVX-induced bone loss was suppressed and uncoupling bone turnover was balanced, as indicated by systemic biomarkers of bone metabolism; no uterine estrogenicity was observed. Moreover, rats administered with Gm-SGP exhibited increased bone mineral density and biomechanical strength and significant restoration of the trabecular microarchitecture compared with rats in the control group. Gm-SGP significantly decreased bone resorption-related indicators in serum. Investigation of the associated mechanisms revealed that Gm-SGP significantly increases the OPG/RANKL ratio at the mRNA and protein levels. Further research suggested that Gm-SGP inhibits the mRNA and protein expressions of important transcription factors of the MAPK and NF-κB signaling pathways. It also attenuates the activation of related transduction signaling pathways by inhibiting phosphorylation of JNK, ERK, p38, and NF-κB, and ultimately suppresses the induction of c-Fos and NFATc1. Overall, these results demonstrate that Gm-SGP inhibits bone resorption by suppressing osteoclastogenesis-related MAPK and NF-κB pathways, thereby improving osteoporosis.
Similar content being viewed by others
References
Alvarez, M. J. M., Neyro, J. L., and Castaneda, S., 2016. Therapeutic holidays in osteoporosis: Long-term strategy of treatment with bisphosphonates. Medicina Clinica, 146(1): 24–29, DOI: https://doi.org/10.1016/j.medcli.2015.03.017.
Anumula, K. R., 1995. Rapid quantitative-determination of sia-lic acids in glycoproteins by high-performance liquid-chro-matography with a sensitive fluorescence detection. Analytical Biochemistry, 230(1): 24–30, DOI: https://doi.org/10.1006/abio.1995.1432.
Asagiri, M., and Takayanagi, H., 2007. The molecular understanding of osteoclast differentiation. Bone, 40(2): 251–264, DOI: https://doi.org/10.1016/j.bone.2006.09.023.
Blair, J. M., Zhou, H., Seibel, M. J., and Dunstan, C. R., 2006. Mechanisms of disease: Roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nature Clinical Practice Oncology, 3(1): 41–49, DOI: https://doi.org/10.1038/ncponc0381.
Boyce, B. F., Xiu, Y., Li, J., Xing, L., and Yao, Z., 2015. NF-κB-mediated regulation of osteoclastogenesis. Endocrinology and Metabolism, 30(1): 35–44.
Boyle, W. J., Simonet, W. S., and Lacey, D. L., 2003. Osteoclast differentiation and activation. Nature, 423(6937): 337–342, DOI: https://doi.org/10.1038/nature01658.
Brar, K. S., 2010. Prevalent and emerging therapies for osteoporosis. Medical Journal Armed Forces India, 66(3): 249–254, DOI: https://doi.org/10.1016/S0377-1237(10)80050-4.
Cheng, B., Li, J., Du, J., Lv, X., Weng, L., and Ling, C., 2012. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-kappaB and MAPKs pathways. Food and Chemical Toxicology, 50(5): 1610–1615, DOI: https://doi.org/10.1016/j.fct.2012.02.019
Cong, Q., Jia, H., Li, P., Qiu, S., Yeh, J., Wang, Y., Zhang, Z., Ao, J., Li, B., and Liu, H., 2017. p38α MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Scientific Reports, 7: 45964.
Cundy, T., Horne, A., Bolland, M., Gamble, G., and Davidson, J., 2007. Bone formation markers in adults with mild osteogene-sis imperfecta. Clinical Chemistry, 53(6): 1109–1114, DOI: https://doi.org/10.1373/clinchem.2006.083055.
Dannemann, C., Gratz, K. W., Riener, M. O., and Zwahlen, R. A., 2007. Jaw osteonecrosis related to bisphosphonate therapy: A severe secondary disorder. Bone, 40(4): 828–834, DOI: https://doi.org/10.1016/j.bone.2006.11.023.
Darnay, B. G., Ni, J., Moore, P. A., and Aggarwal, B. B., 1999. Activation of NF-kappa B by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappa B-inducing kinase-Identification of a novel TRAF6 interaction motif. Journal of Biological Chemistry, 274(12): 7724–7731, DOI: DOI https://doi.org/10.1074/jbc.274.12.7724.
Delaisse, J. M., Engsig, M. T., Everts, V., Ovejero, M. D., Ferreras, M., Lund, L., Vu, T. H., Werb, Z., Winding, B., Lochter, A., Karsdal, M. A., Troen, T., Kirkegaard, T., Lenhard, T., Heegaard, A. M., Neff, L., Baron, R., and Foged, N. T., 2000. Proteinases in bone resorption: Obvious and less obvious roles. Clinica Chimica Acta, 291(2): 223–234, DOI: https://doi.org/10.1016/S0009-8981(99)00230-2.
Doyle, S. L., Jefferies, C. A., and O'Neill, L. A., 2005. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NF-kappa B activation by LPS. Journal of Biological Chemistry, 280(25): 23496–23501.
Eastell, R., O' Neill, T. W., Hofbauer, L. C., Langdahl, B., Reid, I. R., Gold, D. T., and Cummings, S. R., 2016. Postmeno-pausal osteoporosis. Nature Reviews Disease Primers, 2, DOI: https://doi.org/10.1038/Nrdp.2016.69.
Galibert, L., Tometsko, M. E., Anderson, D. M., Cosman, D., and Dougall, W. C., 1998. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. Journal of Biological of Chemistry, 273(51): 34120–34127.
Goel, A., Raghuvanshi, A., Kumar, A., Gautam, A., Srivastava, K., Kureel, J., and Singh, D., 2015. 9-Demethoxy-medicarpin promotes peak bone mass achievement and has bone conserving effect in ovariectomized mice: Positively regulates osteoblast functions and suppresses osteoclastogenesis. Molecular and Cellular Endocrinology, 411: 155–166, DOI: https://doi.org/10.1016/j.mce.2015.04.023.
Huang, H., Ryu, J., Ha, J., Chang, E. J., Kim, H. J., Kim, H. M., Kitamura, T., Lee, Z. H., and Kim, H. H., 2006. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-κB transactivation by RANKL. Cell Death and Differentiation, 13: 1879, DOI: https://doi.org/10.1038/sj.cdd.4401882.
Jimi, E., Aoki, K., Saito, H., D'Acquisto, F., May, M. J., Nakamura, I., Sudo, T., Kojima, T., Okamoto, F., Fukushima, H., Okabe, K., Ohya, K., and Ghosh, S., 2004. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature Medicine, 10(6): 617–624, DOI: https://doi.org/10.1038/nm1054.
Kjesbu, O. S., Taranger, G. L., and Trippel, E. A., 2006. Gadoid mariculture: Development and future challenges - Introduction. Ices Journal of Marine Science, 63(2): 187–191, DOI: https://doi.org/10.1016/j.icesjms.2005.12.003.
Lee, Z. H., and Kim, H. H., 2003. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochemical and Biophysical Research Communications, 305(2): 211–214, DOI: https://doi.org/10.1016/S0006-291x(03)00695-8.
Logar, D. B., Komadina, R., Prezelj, J., Ostanek, B., Trost, Z., and Marc, J., 2007. Expression of bone resorption genes in osteoarthritis and in osteoporosis. Journal of Bone and Mineral Metabolism, 25(4): 219–225, DOI: https://doi.org/10.1007/s00774-007-0753-0.
Lomaga, M. A., Yeh, W. C., Sarosi, I., Duncan, G. S., Furlonger, C., Ho, A., Morony, S., Capparelli, C., Van, G., Kaufman, S., van der Heiden, A., Itie, A., Wakeham, A., Khoo, W., Sasaki, T., Cao, Z., Penninger, J. M., Paige, C. J., Lacey, D. L., Dunstan, C. R., Boyle, W. J., Goeddel, D. V., and Mak, T. W., 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes & Development, 13(8): 1015–1024.
Ma, B., Zhang, Q., Wu, D., Wang, Y. L., Hu, Y. Y., Cheng, Y. P., Yang, Z. D., Zheng, Y. Y., and Ying, H. J., 2012. Strontium fructose 1,6-diphosphate prevents bone loss in a rat model of postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacologica Sinica, 33(4): 479–489, DOI: https://doi.org/10.1038/aps.2011.177.
Min, S. K., Kang, H. K., Jung, S. Y., Jang, D. H., and Min, B. M., 2018. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and os-teoclast differentiation. Cell Death and Differentiation, 25(2): 268–281, DOI: https://doi.org/10.1038/cdd.2017.153.
Morley, P., Whitfield, J. F., and Willick, G. E., 2001. Parathyroid hormone: An anabolic treatment for osteoporosis. Current Pharmaceutical Design, 7(8): 671–687.
Olsen, R. L., Toppe, J., and Karunasagar, L., 2014. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends in Food Science & Technology, 36(2): 144–151, DOI: https://doi.org/10.1016/j.tifs.2014.01.007.
Omelon, S., Georgiou, J., Henneman, Z. J., Wise, L. M., Sukhu, B., Hunt, T., Wynnyckyj, C., Holmyard, D., Bielecki, R., and Grynpas, M. D., 2009. Control of vertebrate skeletal mineralization by polyphosphates. PLoS One, 4(5): e5634, DOI: https://doi.org/10.1371/journal.pone.0005634.
Qiao, H., Wang, T. Y., Yu, Z. F., Han, X. G., Liu, X. Q., Wang, Y. G., Fan, Q. M., Qin, A., and Tang, T. T., 2016. Structural simulation of adenosine phosphate via plumbagin and zole-dronic acid competitively targets JNK/Erk to synergistically attenuate osteoclastogenesis in a breast cancer model. Cell Death & Disease, 7: e2094, DOI: https://doi.org/10.1038/Cddis.2016.11.
Richards, J. B., Rivadeneira, F., Inouye, M., Pastinen, T. M., Soranzo, N., Wilson, S. G., Andrew, T., Falchi, M., Gwilliam, R., Ahmadi, K. R., Valdes, A. M., Arp, P., Whittaker, P., Verlaan, D. J., Jhamai, M., Kumanduri, V., Moorhouse, M., van Meurs, J. B., Hofman, A., Pols, H. A. P., Hart, D., Zhai, G., Kato, B. S., Mullin, B. H., Zhang, F., Deloukas, P., Uitterlinden, A. G., and Spector, T. D., 2008. Bone mineral density, osteoporosis, and osteoporotic fractures: A genome-wide association study. Lancet, 371(9623): 1505–1512, DOI: https://doi.org/10.1016/S0140-6736(08)60599-1.
Rise, M. L., Hall, J. R., Nash, G. W., Xue, X., Booman, M., Katan, T., and Gamperl, A. K., 2015. Transcriptome profiling reveals that feeding wild zooplankton to larval Atlantic cod (Gadus morhua) influences suites of genes involved in oxidation-reduction, mitosis, and selenium homeostasis. Bmc Genomics, 16, DOI: https://doi.org/10.1186/S12864-015-2120-1.
Sharma, S. M., Bronisz, A., Hu, R., Patel, K., Mansky, K. C., Sif, S., and Ostrowski, M. C., 2007. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. Journal of Biological Chemistry, 282(21): 15921–15929, DOI: https://doi.org/10.1074/jbc.M609723200.
Sontag, A., and Krege, J. H., 2010. First fractures among post-menopausal women with osteoporosis. Journal of Bone and Mineral Metabolism, 28(4): 485–488, DOI: https://doi.org/10.1007/s00774-009-0144-9.
Taguchi, T., Seko, A., Kitajima, K., Muto, Y., Inoue, S., Khoo, K. H., Morris, H. R., Dell, A., and Inoue, Y.., 1994. Structural studies of a novel type of pentaantennary large glycan unit in the fertilization-associated carbohydrate-rich glycopeptide isolated from the fertilized eggs of Oryzias latipes. Journal of Biological Chemistry, 269(12): 8762–8771.
Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T., 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell, 3(6): 889–901, DOI: https://doi.org/10.1016/S1534-5807(02)00369-6.
Wada, T., Nakashima, T., Hiroshi, N., and Penninger, J. M., 2006. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends in Molecular Medicine, 12(1): 17–25, DOI: https://doi.org/10.1016/j.molmed.2005.11.007.
Wang, F., Wang, Y., Zhao, Y., Zhan, Q., Yu, P., Wang, J., and Xue, C., 2016. Sialoglycoprotein isolated from eggs of Cara-ssius auratus ameliorates osteoporosis: An effect associated with regulation of the Wnt/beta-catenin pathway in rodents. Journal of Agricultural and Food Chemistry, 64(14): 2875–2882.
Wang, Z. Q., Ovitt, C., Grigoriadis, A. E., Möhle-Steinlein, U., Rüther, U., and Wagner, E. F., 1992. Bone and haematopoietic defects in mice lacking c-fos. Nature, 360(6406): 741–745, DOI: https://doi.org/10.1038/360741a0.
Xia, G. H., Wang, S. S., He, M., Zhou, X. C., Zhao, Y. L., Wang, J. F., and Xue, C. H., 2015. Anti-osteoporotic activity of sia-loglycoproteins isolated from the eggs of Carassius auratus by promoting osteogenesis and increasing OPG/RANKL ratio. Journal of Functional Foods, 15: 137–150, DOI: https://doi.org/10.1016/j.jff.2015.03.021.
Xia, G. H., Yu, Z., Zhao, Y. L., Wang, Y. M., Wang, S. S., He, M., Wang, J. F., and Xue, C. H., 2015. Sialoglycoproteins isolated from the eggs of Carassius auratus prevents osteoporosis by suppressing the activation of osteoclastogenesis related NF-κB and MAPK pathways. Journal of Functional Foods, 17: 491–503, https://doi.org/10.1016/j.jff.2015.05.036.
Zhang, Y., Guan, H. F., Li, J., Fang, Z., Chen, W. J., and Li, F., 2015. Amlexanox suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss. Scientific Reports, 5, DOI: https://doi.org/10.1038/Srep13575.
Zhao, Q., Wang, X., Liu, Y., He, A., and Jia, R., 2010. NFATc1: Functions in osteoclasts. International Journal of Biochemistry & Cell Biology, 42(5): 576–579, DOI: https://doi.org/10.1016/j.biocel.2009.12.018.
Acknowledgments
This study is financially supported by the National Natural Science Foundation of China (No. 31371876).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mao, L., Wang, Y., Wang, M. et al. Sialoglycoproteins Isolated from the Eggs of Gadus morhua Inhibit Bone Resorption in Ovariectomized Rats by Suppressing the MAPK and NF-κB Pathways. J. Ocean Univ. China 18, 1174–1184 (2019). https://doi.org/10.1007/s11802-019-3881-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-019-3881-y