Abstract
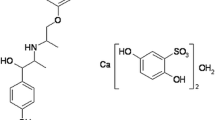
The captopril/Chitosan-gelatin net-polymer microspheres (CTP/CGNPMs) were prepared using Chitosan (CTS) and gelatin (GT) by the methods of emulsification, cross-linked reagent alone or in combination and microcrystalline cellulose (MCC) added in the process of preparation of microspheres, which aimed to eliminate dose dumping and burst phenomenon of microspheres for the improvement of the therapeutic efficiency and the decrease of the side effects of captopril (CTP). The results indicated that CTP/CGNPMs had a spherical shape, smooth surface and integral structure inside but no adhesive phenomena in the preparation. The size distribution ranged from 220 μm to 280 μm. The CTP release test in vitro demonstrated that CTP/CGNPMs played the role of retarding the release of CTP compared with ordinary CTP tablets. The release behaviors of CGNPMS were influenced by preparation conditions such as experimental material ratio (EMR) and composition of cross linking reagents. Among these factors, the EMR (1/4), CLR (FA+SPP) and 0.75% microcrystalline cellulose (MCC) added to the microspheres constituted the optimal scheme for the preparation of CTP/CGNPMs. The ER, DL and SR of CTP/CGNPMs prepared according to the optimal scheme were 46.23±4.51%, 9.95±0.77% and 261±42%, respectively. The CTP/CGNPMs had the good characteristics of sustained release of drug and the process of emulsification and cross-linking were simple and stable. The CGNPMs are likely to be an ideal sustained release formulation for water-soluble drugs.
Similar content being viewed by others
References
Abubakr, O. N., and J. S. Zhang, 2000. Recent progress in sustained/controlled oral delivery of captopril: an overview. Int. J. Pharm., 194: 139–146.
Anaizi, N. H., and C. Swenson, 1993. Instability of captopril solution. Am. J. Hosp. Pharm., 50: 486–488.
Chen, M. X., R. X. Mou, and X. H. Ying, 2004. Determination of residual formaldehyde in grain foods by high-performance liquid chromatography. Chin. J. Anal. Lab., 23: 74–76.
Gallo, J. M., C. T. Hung, and D. G. Perrier, 1984. Analysis of albumin microsphere preparation. Int. J. Pharm., 22: 63–68.
Kawashima, Y., T. Handa, A. Kasai, H. Takenaka, and S. Y. Lin, 1985. Novel method for the preparation of ncontrolled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J. Pharm. Sci., 74: 264–268.
Liu, L. S., A. Y. Thompson, and M. A Heidaran, 1999. An osteoeonducdve collagen/hyaluronate matrix for bone regenerate. Biomaterials, 20: 1097–1108.
Ren, X., X. P. Xu, and L. Y. Liao, 2004. Capillary gas chromatographic determination of seven residual organic solvents in cefoselis hydrogen sulfate and prulixacin. Chin. J. Antibiot., 29: 354–356.
Romankiewjcz, J., 1983. Captopril: an update review of its pharmacological properties and herapeutic efficacy in congestive heart failure. Drugs, 25: 6–21.
Seta, Y., F. Higuchi, Y. Kawahara, K. Nishimura, and R. Okada, 1988. Design and preparation of captopril sustained-release dosage forms and their biopharmaceutical properties. Int. J. Pharm., 41: 245–254.
Shu, X. Z., and K. J Zhu, 2002. Controlled drug release properties of ionically cross-linked chitosan microspheres: the influence of anion structure. Int. J. Pharm., 233: 217–225.
Tian, W. R., Z. Gao, and S. X. Wang, 1993. Studies of captopril of pharmacokinetics on health human body. Chin. Hosp. Pharm. J., 13: 55–56.
Tsai, T., Y. P. San, H. O. Ho, J. S. Wu, and M. T. Sheu, 1998. Film-forming polymer-granulated excipients as the Matrix material for controlled-release dosage forms. J. Control Release, 51: 289–299.
Wang, H., N. Y. Wu, C. Y. Ye, and X. P. Dong, 2004. Determination of seven organic solvents in nicergoline by headspace capillary gas chromatography. Chin. J. Anal., 24: 273–275.
Wang, Y. M., and T. S. Shi, 1996. Process in the studies of controlled release of microsphere formulation. Chin. Pharm. J., 31: 131–134.
Yao, K. D., Y. J. Yin, and M. X. Xu, 1995. Studies of release behavior of chitosan/gelatin net polymer microsphere. Chin. Sci. Bull., 40: 2241–2243.
Zhang, S. Q., Y. Xie, Z. H. Hao, M. Yuan, and G Ling, 1996. Studies of captopril sustained release tablets. Chin. Pharm. J., 31: 668–669.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, L., Xu, J., Song, Y. et al. Preparation and in vitro release performance of sustained-release captopril/Chitosan-gelatin net-polymer microspheres. J Ocean Univ. China 6, 249–254 (2007). https://doi.org/10.1007/s11802-007-0249-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11802-007-0249-5