Abstract
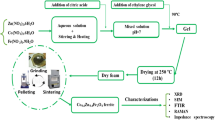
The most remarkable effect in spinel ferrites is the strong dependence of properties on the state of structural disorder and, in particular, on the cation distribution. The structural characterization of a Co-Zn ferrite nanoparticle sample was reported which prepared by wet chemical co-precipitation method. The samples were sintered at three different temperatures viz. 650 °C, 850 °C and 1 050 °C for 12 h. The structural details like: lattice constant and distribution of cations in the tetrahedral and octahedral interstitial voids have been deduced through X-ray diffraction (XRD) data analysis. Lattice constant was found to increase with the increase in Zn2+ ions and sintering temperature. Theoretical intensity ratios of (220), (400), (440) planes were considered, as these reflections are sensitive to cations on the A and B sites. Close agreement of the theoretical intensity ratio with the intensity ratio observed from XRD pattern supports the occupancy of Zn2+ ions and Co2+ ions on the octahedral and tetrahedral sites, respectively.
Similar content being viewed by others
References
GORTER E W. Magnetization in ferrites: Saturation magnetization of ferrites with spinel structure [J]. Nature, 1950, 165(1): 798–800.
CHAE Kwang-pyo, LEE Young-bae, PAIK Seo-wook, LEE Sung-ho. Cation distribution of indium-substituted garnet Y2.4In0.6Fe5O12 [J]. Journal of Applied Physics, 1995, 77(4): 1667–1670.
JADHAV S S, SHIRSATH S E, TOKSHA B G, SHUKLA S J, JADHAV K M. Effect of cation proportion on the structural and magnetic properties of Ni-Zn ferrites nano-size particles prepared by co-Precipitation technique [J]. Chinese Journal of Chemical Physics, 2008, 21(4): 381–386.
MANE D R, BIRAJDAR D D, PATIL S, SHIRSATH S E, KADAM R H. Redistribution of cations and enhancement in magnetic properties of sol-gel synthesized Cu0.7−x CoxZn0.3Fe2O4 (0≤x≤0.5) [J]. Journal Sol-gel Science and Technology, 2011, 58(1): 70–79.
PATANGE S M, SHIRSATH S E, JANGAM G S, LOHAR K S, JADHAV S S, JADHAV K M. Rietveld structure refinement, cation distribution and magnetic properties of Al3+ substituted NiFe2O4 nano particles [J]. Journal of Applied Physics, 2011, 109(5): 053909.
MORIGA T, SAKAMOTO Y, SATO Y, KHALID A H, SUENARI R, NAKA-BAYASHI I. Crystal structures and electrical and optical properties of MgIn2−x GaxO4 solid solutions [J]. Journal of Solid State Chemistry, 1999, 142(1): 206–208.
GONZALEZ C, LOPEZ M L, GAITAN M, VEIGA M L, PICO C. Relationship between crystal structure and electric properties for lithium-containing spinels [J]. Materials Research Bulletin, 1994, 29(8): 903–910.
LAARJ M, KACIM S, GILLOT B. Cationic distribution and oxidation mechanism of trivalent manganese ions in submicrometer MnxCoFe2−x O4 spinel ferrites [J]. Journal of Solid State Chemistry, 1996, 125(1): 67–74.
O’NEILL H S T C, JAMES M, DOLLASE WA, REDFERN S A T. Temperature dependence of the cation distribution in CuAl2O4 spinel [J]. European Journal of Minerals, 2005, 17(4): 581–586.
SMITH J, WIJN H P J. Modern ferrite technology [M]. London: John Wiley, 1959: 140.
RAMANA R A V, RANGA M G, RAVINDER D, BOYANOV B S. High-frequency dielectric behaviour of polycrystalline zinc substituted cobalt ferrites [J]. Journal of Materials Science, 1999, 34(3): 3169–3176.
Wel Qlang Min, Biaoli Jain, Chen Yong Jun. Cation distribution and infrared properties of NixMn1−x Fe2O4 ferrites [J]. Journal of Materials Science, 2001, 36(21): 5115–5118.
TRIVEDI Bimal S, KULKARNI R G. Mössbauer and magnetization study of cobalt ferrite aluminates [J]. Solid State Communications, 1993, 86(5): 327–331.
GHATAGE A K, PATIL S A, PARANJPE S K. Neutron diffraction study of chromium substituted nickel ferrite [J]. Solid State Communications, 1996, 98(10): 885–888.
BERTAUT E F. On ferrites, theory and practice [J]. J Phys Radium, 1950, 11(5): 208–212.
FURUHASHI H, INAGAKI M, NAKA S. Determination of cation distribution in spinels by X-ray diffraction method [J]. Journal of Inorganic Chemistry, 1973, 35(8): 3009–3014.
SHINDE S S, JADHAV K M. Electrical and dielectric properties of silicon substituted cobalt ferrites [J]. Materials Letters, 1998, 37(1/2): 63–67.
CULLITY B D. Elements of X-ray diffraction [M]. Boston: Addison-Wesley Publishing Company Inc, 1956: 352.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamble, S.R., Shirsath, S.E., Patange, S.M. et al. Sintering temperature reflected cation distribution of Zn2+ substituted CoFe2O4 . J. Cent. South Univ. 20, 1469–1474 (2013). https://doi.org/10.1007/s11771-013-1636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-013-1636-2