Abstract
Objectives
Because chemoradiotherapy using cisplatin and S-1, an oral fluoropyrimidine, is effective for unresectable non-small cell lung cancer (NSCLC), an induction setting was used in a multicenter phase II study (Clinical trial number: UMIN000008205). The correlations of relapse and clinicopathological factors were analyzed.
Methods
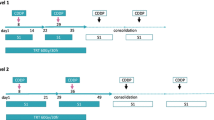
We defined locally advanced NSCLC as pathologically proven chest wall invasion or hilar and/or mediastinal lymph node metastases by endobronchial ultrasound-guided transbronchial needle aspiration. The patients received two courses of S-1 administration for 14 days and intravenous cisplatin injection on day 8. A total dose of 40 Gy radiotherapy was concurrently received. Surgical resection was performed after completion of the treatment.
Results
Of the 23 eligible patients, 18 had stage IIIA and 5 had stage IIB NSCLC. Twenty of the eligible patients (87.0%) completed the regimen. Six (26.1%) complete responses were identified and 12 cases (52.2%) were histopathologically downstaged by induction chemoradiotherapy (ICRT). The 3-year overall survival rate was 58.1% and relapse-free survival (RFS) rate was 52.0%, respectively. Among several clinicopathological parameters, univariate RFS analysis identified that only downstaging was significantly associated with longer RFS times (p = 0.003). The radiological response did not reflect pathological response. When the variables of preoperative pathologically proven N2 metastasis, pathological ICRT effectiveness, and downstaging were included in the Cox proportional hazard modes, only the parameter of downstaging displayed significant hazard ratio (hazard ratio 0.13, p = 0.010).
Conclusion
This protocol is considered an option among preoperative therapies and has obvious benefits for pathologically downstaged cases.
Clinical trial number
UMIN000008205.
Trial registration date
June 19, 2012.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14.
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura KFM. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Shirasaka T, Shimamoto Y, Fukushima M, Shi Y. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–09.
Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsui K, Kudoh S, et al. Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer. 2001;85:939–43.
Ichinose Y, Yoshimori K, Sakai H, Nakai Y, Sugiura T, Kawahara M, et al. S-1 plus cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer: a multi-institutional phase II trial. Clin Cancer Res. 2004;10:7860–4.
Ohyanagi F, Yamamoto N, Horiike A, Harada H, Kozuka T, Murakami H, et al. Phase II trial of S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Br J Cancer. 2009;101:225–31.
Nogami N, Takigawa N, Hotta K, Segawa Y, Kato Y, Kozuki T, et al. A phase II study of cisplatin plus S-1 with concurrent thoracic radiotherapy for locally advanced non-small-cell lung cancer: the Okayama Lung Cancer Study Group Trial 0501. Lung Cancer. 2015;87:141–47.
Tsuchiya T, Nakamura Y, Hayashi N, Matsumoto K, Miyazaki T, Nakatomi K, et al. A multicenter phase II study of concurrent chemoradiotherapy with cisplatin and oral S-1, followed by surgery for locally advanced non-small-cell lung cancer. Cancer Treat Commun. 2016;7:11–6.
Eisenhauer E, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86.
Caglar HB, Baldini EH, Othus M, Rabin MS. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer. 2009;115:4156–66.
Taira N, Kawasaki H, Furugen T, Ichi T, Kushi K, Yohena T, et al. The long-term prognosis of induction chemotherapy followed by surgery for N2 non-small cell lung cancer: a retrospective case series study. Ann Med Surg. 2017;17:65–9.
Robinson LA, Tanvetyanon T, Grubbs D, Antonia S, Creelan B, Fontaine J, et al. Induction chemoradiotherapy versus chemotherapy alone for superior sulcus lung cancer. Lung Cancer. 2018;122:206–13.
Vokes EE, Weichselbaum RR. Concomitant chemoradiotherapy: rationale and clinical experience in patients with solid tumors. J Clin Oncol. 1990;8:911–34.
Lawrence TS, Blackstock W, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21.
Naida JD, Davis MA, Lawrence TS. The effect of activation of wild-type p53 function on fluoropyrimidine-mediated radiosensitization. Int J Radiat Oncol Biol Phys. 1998;41:675–80.
Takagi M, Sakata KI, Someya M, Tauchi H, Iijima K, Matsumoto Y, et al. Gimeracil sensitizes cells to radiation via inhibition of homologous recombination. Radiother Oncol. 2010;96:259–66.
Schwartz GN, Teicher B, Eder JP, Korbut T, Holden S, Ara G, et al. Metabolic basis of the synergistic antitumor activities of 5-fluorouracil and cisplatin in rodent tumor models in vivo. Cancer Chemother Pharmacot. 1993;32:167–72.
Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–25.
Yamamoto N, Yamanaka T, Ichinose Y, Kubota K, Sakai H, Gemma A, et al. Pooled analysis of S-1 trials in non-small cell lung cancer according to histological type. Anticancer Res. 2010;30:2985–90.
Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–91.
Tsuchiya T, Arai J, Matsumoto K, Miyazaki T, Honda S, Tagawa T, et al. Prognostic Impact of the ABCC11/MRP8 polymorphism in adjuvant oral chemotherapy with S-1 for non-small cell lung cancer. Chemotherapy. 2016;61:77–86.
Jeremić B, Casas F, Dubinsky P, Gomez-Caamano A, Čihorić N, Videtic G, et al. Treatment-related predictive and prognostic factors in trimodality approach in stage IIIA/N2 non-small cell lung cancer. Front Oncol. 2018;8:30. https://doi.org/10.3389/fonc.2018.00030 (eCollection).
Phernambucq ECJ, Spoelstra FOB, Paul M, Senan S, Melissant CF, Postmus PE, et al. Evaluation of a treatment strategy for optimising preoperative chemoradiotherapy in stage III non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:1052–57.
Shintani Y, Funakoshi Y, Inoue M, Takeuchi Y, Okumura M, Maeda H, et al. Pathological status of mediastinal lymph nodes after preoperative concurrent chemoradiotherapy determines prognosis in patients with non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2012;18:530–35.
Acknowledgements
We wish to thank Dr. Sumihisa Honda and Dr. Shuntaro Sato for providing statistical advice. We also thank Dr. Mary Durbin for critical reading of the manuscript.
Funding
No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclosure with regard to commercial support. The authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsuchiya, T., Matsumoto, K., Miyazaki, T. et al. Concurrent chemoradiotherapy using cisplatin and S-1, followed by surgery for stage II/IIIA non-small cell lung cancer. Gen Thorac Cardiovasc Surg 67, 537–543 (2019). https://doi.org/10.1007/s11748-018-01058-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-018-01058-3