Abstract
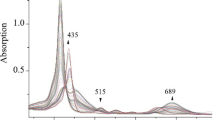
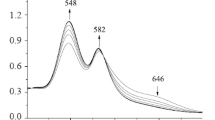
By using mixed-solvents method, five kinds of p-substituted tetraphenylporphyrin compounds [T(p-R)PPH2, R=NO2, Cl, CH3, OCH3, OH] were synthesized by the condensation of p-substituted benzaldehyde with pyrrole in mixed solvents (propionic acid, acetic acid and nitrobenzene), and corresponding ferric complexes [T(p-R)PPFeIIICl] were synthesized in dimethylformamide. The above free base porphyrins were obtained in 30%–50% yields, metalation yields were up to 90% and total yields of ferric complexes were 27%–50%. Effects of reactive conditions, solvents and oxidants on yields of free base porphyrins were investigated and the relevant mechanism was discussed. Structures of the above porphyrin complexes were characterized by ultravioletvisible (UV-Vis), infrared (IR) and far infrared (FIR) spectroscopy.
Similar content being viewed by others
References
Haber J, Matachowski L, Pamin K, Poltowicz J. The effect of peripheral substituents in metalloporphyrins on their catalytic activity in Lyons system. J Mol Catal A Chem, 2003, 198(1–2): 215–221
Tagliatesta P, Pastorini A. Remarkable selectivity in the cyclopropanation reactions catalysed by an halogenated iron meso-tetraphenylporphyrin. J Mol Catal A Chem, 2003, 198(1–2): 57–61
Zakharieva O, Trautwein A X, Veeger C. Porphyrin-Fe(III)-hydroperoxide and porphyrin-Fe(III)-peroxide anion as catalytic intermediates in cytochrome P450-catalyzed hydroxylation reactions: a molecular orbital study. Biophysical Chemistry, 2000, 88(1–3): 11–34
Davydov R, Makris T M, Kofman V, Werst D E, Sligar S G, Hoffman B M. Hydroxylation of camphor by-reduced oxycytochrome P450cam: Mechanistic implications of EPR and ENDOR studies of catalytic intermediates in native and mutant enzymes. J Am Chem Soc, 2001, 123(7): 1403–1415
Guo C C, Liu Q, Wang X T, Hu H Y. Selective liquid phase oxidation of toluene with air. Applied Catalysis A-General, 2005, 282(1–2): 55–59
Yuan Y, Ji H B, Chen Y X, Han Y, Song X F, She Y B, Zhong R G. Oxidation of cyclohexane to adipic acid using Fe-porphyrin as a biomimetic catalyst. Org Process Res Dev, 2004, 8(3): 418–420
Wang L Z, She Y B, Zhong R G, Ji H B, Zhang Y H, Song X F. A green process for oxidation of p-nitrotoluene Catalyzed by metalloporphyrins under mild conditions. Org Process Res Dev, 2006, 10(4), 757–761
Zhang R, Horner J H, Newcomb M. Laser flash photolysis generation and kinetic studies of porphyrin-manganese-oxo intermediates. Rate constants for oxidations effected by porphyrin-MnV-oxo species and apparent disproportionation equilibrium constants for porphyrin-MnV-oxo species. J Am Chem Soc, 2005, 127(18): 6573–6582
She Y B, Feng L S, Wang A X, Li X Y. Synthesis of substituted μ-oxo-bis[tetra-phenyl porphyrinatoiron] compounds from free base porphyrins by a one-pot method. Chin J Chem Eng, 2008, 16(3): 369–372
Geier G R, Ciringh Y Z, Li F R, Haynes D M, Lindsey J S. Two-step, one-flask syntheses of meso-substituted porphyrinic macrocycles. Org Lett, 2000, 2(12): 1745–1748
Sharada D S, Muresan A Z, Muthukumaran K M, Lindsey J S. Direct synthesis of palladium porphyrins from acyldipyrromethanes. J Org Chem, 2005, 70(9): 3500–3510
Adler A D, Longo F R, Shergalis W. Mechanistic investigations of porphyrin syntheses. I. Preliminary studies on ms-tetraphenylporphin. J Am Chem Soc, 1964, 86(15): 3145–3149
Sun Z C, She Y B, Li X Y, Yu Y M, Zhong R G. Synthesis of Tetraphenylporphyrin and its influent factors. Chin J Appl Chem, 2007, 24(suppl): 584–586
Kristine P, Thomas G S. Core expansion, ruffling, and doming effects on metalloporphyrin vibrational frequencies. J Am Chem Soc, 1992, 114(10): 3793–3801
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., She, Y. & Zhong, R. Synthesis of p-substituted tetraphenylporphyrins and corresponding ferric complexes with mixed-solvents method. Front. Chem. Eng. China 3, 457–461 (2009). https://doi.org/10.1007/s11705-009-0169-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-009-0169-6