Abstract
Background
Enhanced recovery after surgery (ERAS) programmes are evidence-based care improvement processes for surgical patients, which are designed to decrease the impact the anticipated negative physiological cascades following surgery.
Aim
To perform a systematic review and meta-analysis of randomised clinical trials (RCTs) to evaluate the impact of ERAS protocols on outcomes following bariatric surgery compared to standard care (SC).
Methods
A systematic review was performed in accordance with PRISMA guidelines. Meta-analysis was performed using Review Manager version 5.4
Results
Six RCTs including 740 patients were included. The mean age was 40.2 years, and mean body mass index was 44.1 kg/m2. Overall, 54.1% underwent Roux-en-Y gastric bypass surgery (400/740) and 45.9% sleeve gastrectomy (340/700). Overall, patients randomised to ERAS programmes had a significant reduction in nausea and vomiting (odds ratio (OR): 0.42, 95% confidence interval (CI): 0.19–0.95, P = 0.040), intraoperative time (mean difference (MD): 5.40, 95% CI: 3.05–7.77, P < 0.001), time to mobilisation (MD: − 7.78, 95% CI: − 5.46 to − 2.10, P < 0.001), intensive care unit stay (ICUS) (MD: 0.70, 95% CI: 0.13–1.27, P = 0.020), total hospital stay (THS) (MD: − 0.42, 95% CI: − 0.69 to − 0.16, P = 0.002), and functional hospital stay (FHS) (MD: − 0.60, 95% CI: − 0.98 to − 0.22, P = 0.002) compared to those who received SC.
Conclusion
ERAS programmes reduce postoperative nausea and vomiting, intraoperative time, time to mobilisation, ICUS, THS, and FHS compared to those who received SC. Accordingly, ERAS should be implemented, where feasible, for patients indicated to undergo bariatric surgery.
Trial registration International Prospective Register of Systematic Reviews (PROSPERO – CRD42023434492.
Graphical Abstract

Similar content being viewed by others
Introduction
In the western world, the incidence of obesity has increased nearly twofold over the past four decades [1], generating perceptions that the prevalence has reached ‘epidemic proportions’ [2]. Furthermore, it is now estimated that 70% of the adult population are now considered to live with overweight or obesity [1, 3, 4]. Medical therapies, such as glucagon-like peptide-1 receptor agonists, have demonstrated promise in achieving substantial weight loss in patients with obesity [5], as well as significant cardiovascular improvements; however, the response to medication is variable and a significant group of patients do not tolerate the medication due to side effects [6]. The results are impressive, albeit short term, and the treatment is likely required long term for maintenance of effect.
Surgical treatment of obesity remains the most effective and durable option for both significant weight loss and control or prevention of metabolic diseases such as type 2 diabetes mellitus (T2DM) [7, 8] and prevention of a range of malignancies [9, 10]. Moreover, patients who undergo bariatric surgery have significant improvements in their all-cause mortality rates and overall life expectancy compared to those who do not undergo surgery [11]. Accordingly, the pragmatism of using bariatric surgery to improve and optimise patient health has come into vogue, which has subsequently been reciprocated in the dramatic increase in the number of such procedures being performed across Europe and the USA in recent years [12, 13].
Postoperative outcomes following bariatric surgery have improved significantly in recent times [14]. This is likely due to widespread adoption of minimally invasive approaches [15, 16], the centralisation of bariatric surgery to specialised bariatric units [17], and the overall increase in patient volume which has translated into enhanced clinical outcomes [15]. Notwithstanding these advances in surgical care, the risk of mortality following bariatric surgery is far from negligible [18], in particular in cases who are considered to be ‘high risk’ through comorbidity or poor physiological reserve [19]. Enhanced recovery after surgery (ERAS) protocols are an evidence-based care improvement process for surgical patients which have been designed to negate the impact of the anticipated physiological and immunological cascade patients are subject to following major surgery [20]. ERAS protocols have been associated with reduced post-operative complications, inpatient hospitalisation duration, and hospital costs [20, 21]. Following the robust implementation and success of ERAS protocols in other surgical specialities (including thoracic [22], urological [23], and colorectal surgeries [24]), ERAS protocols have now been incorporated into the management paradigm for patients undergoing bariatric surgery, with results from observational and randomised clinical trial (RCT) data suggesting there may be benefit in postoperative outcomes expected following effective ERAS implementation [25,26,27,28]. Consequently, the ERAS Society published recommended preadmission, pre-, intra-, and post-operative ERAS guidelines in 2021 to encourage implementation and adoption of these protocols for perspective patients due to undergo bariatric surgery [29].
While previous meta-analyses have been performed and demonstrate the positive effect of ERAS protocols on outcomes following bariatric surgery [30,31,32], these studies are limited due to a reliance upon observational and retrospective data to decipher the potential benefit of ERAS implementation. Therefore, a meta-analysis consisting solely of RCTs is necessary to determine the value of ERAS protocols on patients undergoing bariatric surgery. Accordingly, the aim of this study was to perform a systematic review and meta-analysis of RCTs to evaluate the impact of ERAS protocols on patient outcomes following bariatric surgery.
Methods
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [33]. Ethical approval was not required from the local institutional review board due to this study using data from previously published resources. All authors contributed to formulating the study protocol, and it was then registered with the International Prospective Register of Systematic Reviews (PROSPERO – CRD42023434492).
Population, Intervention, Comparison, Outcome (PICO) Tool
Applying the PICO framework [34], the clinical research question the authors wished to address was as follows:
Population — Any patients indicated to undergo bariatric surgery in a randomised clinical trial setting.
Intervention — Any patients who were randomised to undergo ERAS.
Comparison — Any patients who were randomised to standard care (SC).
Outcomes — The study outcomes included: Overall complications, major complications, leaks, bleeding, surgical site infections (SSIs), nausea and vomiting, reoperation, intraoperative time (measured in minutes), postoperative pain, time to mobilisation, duration of intensive care unit stay (ICUS), total hospital stay (THS), functional hospital stay (FHS), 30-day readmissions, hospitalisation costs, and mortality.
Search Strategy
An electronic search was performed of the PubMed, EMBASE and Cochrane (CENTRAL) databases on the 12th May 2023 for relevant RCTs which would be suitable for inclusion in this study. The search was performed of all fields under the following headings: (enhanced recovery after surgery) and (bariatric surgery) which were linked by the Boolean operator, ‘AND’. Included studies were limited to those published in the English language and of prospective randomised design. Studies were not restricted based on year of publication. For retrieved studies, their titles were initially screened, before the abstracts and full texts which were deemed appropriate were reviewed.
Inclusion and Exclusion Criteria
Studies were considered for inclusion in the current study if they met the following inclusion criteria: (1) studies had to be prospective RCTs which randomised adult patients aged 18 years or older indicated to undergo bariatric surgery to ERAS or SC protocols; (2) studies had to include surgical outcomes following ERAS and SC. Studies were excluded from this study if they failed to meet the above inclusion criteria.
Definitions
-
Intensive care unit stay — time measured in hours from the end of the surgery until discharge criteria from the ICU were met.
-
Functional hospital stay — time measured in hours from the end of the surgery until discharge criteria had been met, as described by Geubells et al. [28].
-
Total hospital stay — time measured in hours from the end of the surgery until actual time of discharge from hospital, as described by Geubells et al. [28].
-
Overall complications — all complications, as measured using the Clavien-Dindo classification for surgical complications [35]
-
Major complications — complications of grade ≥ 3, as measured using the Clavien-Dindo classification for surgical complications [35]
-
Postoperative pain — measured using the visual analogue scale [36]
Data Extraction and Quality Assessment
The literature search was performed by two independent reviewers (M.G.D and N.E.D.) using a predesigned search strategy. Duplicate studies were manually removed. Each reviewer then reviewed the titles, abstracts, and/or full texts of the retrieved manuscripts to ensure all inclusion criteria was met, before extracting the following data: (1) first author name, (2) year of publication, (3) study design (including intervention and control), (4) country of research facility, (5) number of patients indicated to undergo bariatric surgery, (6) number of patients randomised to ERAS and SC protocols, and (7) surgical data (as outlined in the PICO framework). Risk of bias and methodological assessment of included studies was undertaken using the Risk of Bias 2.0 assessment for RCTs.
Statistical Analysis
Descriptive statistics were used to determine the associations between ERAS and SC with surgical outcomes (Fisher’s exact test, †) [37]. Thereafter, outcomes for patients randomised to ERAS and SC were expressed as dichotomous or continuous outcomes, reported as odds ratios (ORs) with their corresponding 95% confidence intervals (CIs), following estimation using the Mantel–Haenszel method. Either fixed or random effects models were applied on the basis of whether significant heterogeneity (I2 > 50%) existed between studies included in the analysis. All tests of significance were two-tailed with P < 0.05 indicating statistical significance. Descriptive statistics were performed using the Statistical Package for Social Sciences (SPSS) version 26 (International Business Machines Corporation, Armonk, New York). Meta-analysis was performed using Review Manager (RevMan), Version 5.4 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
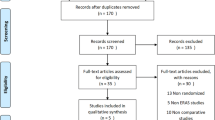
Literature Search and Study Characteristics
The systematic search strategy identified a total of 502 studies, of which 182 duplicate studies were manually removed. The remaining 320 studies were screened for relevance, after which 10 had their full texts reviewed. In total, 6 RCTs met the eligibility criteria and were included in this systematic review and meta-analysis (Fig. 1). Of the 6 RCTs included in this analysis, all studies reported outcomes in relation to patients indicated to undergo bariatric surgery and who were randomised to ERAS or SC pathways (100.0%, 6/6). Publication dates of included studies ranged from 2013 to 2022. Study data, inclusion criteria, and risk of bias assessments for the 6 included RCTs are in Table 1. Breakdown of the components of each ERAS protocol from each included RCT is outlined in Table 2.
Clinicopathological and Surgical Characteristics
In total, data from 740 patients was included. The mean age at diagnosis was 40.2 years. The majority of patients were female (75.6%, 517/684). The mean body weight was 122.9 kg. The mean body mass index (BMI) was 44.1 kg/m2. Overall, 54.1% of patients underwent Roux-en-Y gastric bypass surgery (GBS) (400/740) and 45.9% underwent sleeve gastrectomy (340/700). In total, 50.1% of patients were randomised to undergo ERAS (371/740) and 49.9% to SC (369/740). A detailed breakdown of data from included studies is outlined in Table 3.
Overall Complication Rates
When analysing data from the 6 included RCTs, the overall complication rate was 11.8% (87/740). There was no significant difference observed in the overall complication rate for ERAS candidates compared to those who received SC (ERAS: 11.9% (44/371) vs. SC: 11.7% (43/369), P = 1.000, †) (Table 4). At meta-analysis, there was no significant difference observed in the overall complication rate for ERAS patients compared to those who received SC (OR: 1.07, 95% CI: 0.68–1.71, P = 0.760) (Fig. 2A).
Major Complication Rates
In total, 5 RCTs reported major complication rates, identifying a major complication rate of 3.4% (19/560). There was no significant difference observed in the major complication rate for ERAS candidates compared to those who received SC (ERAS: 3.6% (10/281) vs. SC: 3.2% (9/279), P = 1.000, †) (Table 4). At meta-analysis, there was no significant difference observed in the major complication rate for ERAS candidates compared to those who received SC (OR: 1.08, 95% CI: 0.44–2.69, P = 0.860) (Fig. 2B).
Leaks
Four RCTs reported leak rates. Overall, there was a leak rate of 1.5% (6/392). There was no significant difference observed in the leak rate for ERAS patients compared to those who received SC (ERAS: 1.5% (3/196) vs. SC: 1.5% (3/196), P = 1.000, †) (Table 4). At meta-analysis, there was no significant difference observed in the leak rate for ERAS patients compared to those who received SC (OR: 0.97, 95% CI: 0.19–4.93, P = 0.970) (Fig. 2C). Geubbels et al. were the only study reporting on intra-operative leak testing to assess anastomotic or staple line integrity, where methylene blue dye was used [28]. None of the included studies reported post-operative contrast swallow assessments.
Bleeding
Four RCTs reported bleeding rates. Overall, there was a bleeding rate of 1.5% (6/390), which included 4 staple-line bleeds, 1 upper gastrointestinal bleed, and 1 splenic laceration. There was no significant difference observed in the bleeding rate for ERAS patients compared to those who received SC (ERAS: 1.5% (3/196) vs. SC: 1.5% (3/194), P = 1.000, †) (Table 4). At meta-analysis, there was no significant difference observed in the bleeding rate for ERAS patients compared to those who received SC (OR: 0.97, 95% CI: 0.19–4.93, P = 0.970) (Fig. 2D).
Surgical Site Infection
Pimenta et al. were the only RCT that reported SSI rates. No patients developed SSIs in this study (0.0%, 0/20). Accordingly, descriptive statistical and meta-analyses were incalculable.
Nausea and Vomiting
Three RCTs reported nausea and vomiting rates. Overall, there was an overall incidence of nausea and vomiting of 9.9% (30/312). There was a non-significant difference observed in nausea and vomiting rates for ERAS patients compared to those who received SC (ERAS: 6.4% (10/156) vs. SC: 13.5% (21/156), P = 0.056, †) (Table 4). At meta-analysis, there was a significant difference observed in the nausea and vomiting rates for ERAS patients compared to those who received SC (OR: 0.42, 95% CI: 0.19–0.95, P = 0.040) (Fig. 2E).
Reoperation Rates
In total, 4 RCTs reported results in relation to reoperation, which demonstrated a reoperation rate of 0.7% (3/442). There was no significant difference observed in reoperation rates for ERAS patients compared to those who received SC (ERAS: 0.5% (1/221) vs. SC: 0.9% (2/221), P = 1.000, †) (Table 4). At meta-analysis, there was no significant difference observed in the reoperation rate for ERAS patients compared to those who received SC (OR: 0.49, 95% CI: 0.04–5.55, P = 0.570) (Fig. 3A).
Intraoperative Time
Three RCTs reported intraoperative time for patients subject to ERAS and SC protocols. At meta-analysis, there was significant increased observed in the intraoperative time for SC patients compared to ERAS (MD: 5.40, 95% CI: 3.05–7.77, P < 0.001) (Fig. 3B).
Postoperative Pain
Four RCTs reported postoperative pain using the visual analogue scale (VAS). At meta-analysis, there was a non-significant difference observed in postoperative pain for ERAS patients compared to those who received SC (MD: − 0.49, 95% CI: − 1.75–0.77, P = 0.450) (Fig. 3C).
Time to Mobilisation
Two RCTs reported time to mobilisation for patients subject to ERAS and SC protocols. At meta-analysis, there was significant reduction in time to mobilisation for ERAS patients compared to SC (MD: − 3.78, 95% CI: − 5.46 to − 2.10, P < 0.001) (Fig. 3D).
Intensive Care Unit Stay
Two RCTs reported outcomes in relation to ICUS. At meta-analysis, there was significant reduction in ICUS times for ERAS patients compared to SC (MD: 0.70, 95% CI: 0.13–1.27, P = 0.020) (Fig. 3E).
Total Hospital Stay
Five RCTs reported outcomes in relation to THS. At meta-analysis, there was a significant reduction in THS for ERAS patients compared to those who received SC (MD: − 0.42, 95% CI: − 0.69 to − 0.16, P = 0.002) (Fig. 4A).
Functional Hospital Stay
Three RCTs reported outcomes in relation to FHS. At meta-analysis, there was a significant reduction in FHS for ERAS patients compared to those who received SC (MD: − 0.60, 95% CI: − 0.98 to − 0.22, P = 0.002) (Fig. 4B).
30-Day Readmissions
All 6 RCTs reported 30-day readmission rates. Overall, there was a 30-day readmission rate of 3.8% (28/740). There was no significant difference observed in the 30-day readmission rate for those ERAS patients compared to SC (ERAS: 11.9% (15/371) vs. SC: 11.7% (13/369), P = 0.848, †) (Table 4). At meta-analysis, there was no significant difference observed in the 30-day readmission rate for ERAS patients compared to SC (OR: 1.29, 95% CI: 0.63–2.65, P = 0.490) (Fig. 4C).
Hospitalisation Costs
Lemanu et al. were the only RCT reporting hospitalisation costs. At meta-analysis, there was a non-significant reduction in costs for ERAS patients compared to those who received SC (MD: − 427.00, 95% CI: − 3841.03–2987.03, P = 0.810) (Fig. 4D).
Mortality Rates
Four of the included RCTs reported mortality rates. No patients died during these studies (0.0%, 0/740). Accordingly, descriptive statistical and meta-analyses were incalculable.
Discussion
This systematic review and meta-analysis integrated data from six prospective, randomised studies which evaluated the impact of ERAS protocols on both peri- and postoperative clinical outcomes following bariatric surgery. Overall, 740 patients (vastly representative of real-world patients undergoing bariatric surgery) were included, and the results from this meta-analyses demonstrate favourable clinical outcomes for those who were randomised to ERAS protocols (compared to SC) when undergoing bariatric surgery. While these data support the results of previous reviews [30, 32, 38], this study provides novelty through combining the highest possible quality evidence to demonstrate the significant benefits which may be anticipated through robust ERAS adherence when performing bariatric surgery. Moreover, this review incorporates recent data from bariatric units from all over the world, providing a congruent message in support of using ERAS protocols as a strategy to improve outcomes, irrespective of cultural exposure, healthcare (in)equity, or the genetic composition of the local population in question. Thus, this data supports implementation of ERAS protocols for suitable candidate patients indicated to undergo bariatric surgery, where feasible.
As outlined, these results support the routine utility of ERAS protocols in primary bariatric surgery where feasible. This is due to a significant reduction being observed in the proportion of patients experiencing nausea and vomiting, coupled with significant reduction in intraoperative duration, time to mobilisation, ICUS, THS, and FHS, while not increasing complications or readmission rates as a consequence. In tandem, with reduced hospital and ICU stays, these results suggest positive implications for patient health, quality of life metrics, and clinical outcomes following bariatric surgery [39]. Thus, these results demonstrate the potential cost effectiveness of using ERAS protocols in day-to-day clinical practice, albeit not directly demonstrated in the results of this rudimentary systematic review. In essence, reduced intra-operative time, ICUS, THS, and FHS will have positive implications on the cost-effectiveness of the provision of bariatric services are significant when attempting to optimise healthcare economies across the globe, particularly as we move towards ‘fast track’ and’23-h’ hospital discharges following bariatric surgery [40, 41]. For example, several previous high-quality studies have illustrated the cost-effectiveness of ERAS in other surgical specialties, including those reporting outcomes following hepatobiliary [42], colorectal [24], and thoracic resections [22], which is secondary to an overall reduction in patient morbidity and hospital stay, when ERAS has been diligently implemented and adhered to [43, 44]. Furthermore, if such measures were adopted in the setting of high-volume bariatric-dedicated high dependency units, costs could theoretically be further extenuated through increased efficiency and experience in managing these patients post-operatively [45]. Albeit this cost-effectiveness was not directly observed in the results in the current review, this shortcoming is most plausibly explained by a type II statistical error [46], which is due to results from just 1 RCT (where n = 78) being integrated and analysed for this outcomes measure. Therefore, it is plausible that ERAS following bariatric surgery has similar cost-effectiveness to that noted in other surgical specialties [22, 24, 42], as has been previously demonstrated in observational studies in bariatric surgery [47].
Traditionally, enhancing surgical outcomes focused primarily upon advancing technological innovations, the centralisation of specialised (complex or revisional) cases, and increasing the adoption of checklists to ensure the provision of consistently high-quality care [48,49,50]. These timely changes have improved patient outcomes; however, providing benchmark care to the increasing numbers of people living with obesity creates several surgical and anaesthetic challenges, which must be combatted by pre-empting the physiological and immunogenic sequelae that follow surgical manipulation of the upper gastrointestinal tract. Importantly, the data explored in this meta-analysis of RCTs supports the pragmatism of implementing ERAS protocols in this unique and increasingly more common patient cohort, though the illustration of promise these simple adjustments have in further optimising patients prior to and during bariatric surgery.
Despite several strengths, the authors acknowledge that this study is subject to several unavoidable limitations. Firstly, as stands true for the majority of RCTs performed in the field of surgery, none of the included RCTs in this analysis were ‘blinded’. The inability to blind surgeons to interventions leads to these RCTs to be classed as ‘open label’, making them subject to unintentional biases [51]. Secondly, it is plausible that certain potential confounders (for example, patient age, patient comorbidities, surgeons experience) may influence the results of this study, and the authors also acknowledge the measurement of the severity of morbidities and clinical conditions triggering readmission are not measured in the current analysis. Thirdly, none of the included studies provided data surrounding patient reported perspectives in relation to ERAS protocols following bariatric surgery, thus limiting subjective insights this study provides into the impact of ERAS on the patient experience of bariatric surgery. Finally, while data was compiled from all available RCTs published on this topic, this meta-analysis relies on data from just 740 patients, limiting the robustness of results. Despite these limitations, this meta-analysis of RCTs provides comprehensive, high-quality analyses which support the implementation of ERAS protocols to bariatric surgical practice, where feasible.
In conclusion, this systematic review and meta-analysis of RCT data demonstrates the clinical utility of ERAS protocols in reducing post-operative nausea and vomiting, time to ambulation, ICUS, FHS, and THS. Based on the results of this study, we advocate for the routine implementation of ERAS protocols to bariatric surgical units, where feasible. Thus, the next generation of prospective randomised clinical trials should focus on refining and adjusting our approach to ERAS following bariatric surgery, in order to further improve outcomes for patients undergoing bariatric surgery.
Data availability
Data will be made available upon reasonable request from the corresponding author.
References
Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377(9765):557–67.
Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30(3):415–44.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Sanchis-Gomar F, Lavie CJ, Mehra MR, et al. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–53.
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002.
Ryan DH, Lingvay I, Colhoun HM, et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–9.
Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation. 2021;143(15):1468–80.
Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol (Lausanne). 2019;10:626.
Davey MG, Ryan OK, Ryan É J, et al. The impact of bariatric surgery on the incidence of colorectal cancer in patients with obesity-a systematic review and meta-analysis of registry data. Obes Surg. 2023;33(8):2293–302.
Aminian A, Wilson R, Al-Kurd A, et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA. 2022;327(24):2423–33.
Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830–41.
Alalwan AA, Friedman J, Park H, et al. US national trends in bariatric surgery: a decade of study. Surgery. 2021;170(1):13–7.
Fort JM, Vilallonga R, Lecube A, et al. Bariatric surgery outcomes in a European Centre of Excellence (CoE). Obes Surg. 2013;23(8):1324–32.
le Roux CW, Heneghan HM. Bariatric surgery for obesity. Med Clin North Am. 2018;102(1):165–82.
Markar SR, Santoni G, Holmberg D, et al. Bariatric surgery volume by hospital and long-term survival: population-based NordOSCo data. Br J Surg. 2023;110(2):177–82.
Banka G, Woodard G, Hernandez-Boussard T, et al. Laparoscopic vs open gastric bypass surgery: differences in patient demographics, safety, and outcomes. Arch Surg. 2012;147(6):550–6.
El Chaar M, Claros L, Ezeji GC, et al. Improving outcome of bariatric surgery: best practices in an accredited surgical center. Obes Surg. 2014;24(7):1057–63.
Robertson AGN, Wiggins T, Robertson FP, et al. Perioperative mortality in bariatric surgery: meta-analysis. Br J Surg. 2021;108(8):892–7.
Morino M, Toppino M, Forestieri P, et al. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246(6):1002–7.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–8.
Fair LC, Leeds SG, Whitfield EP, et al. Enhanced recovery after surgery protocol in bariatric surgery leads to decreased complications and shorter length of stay. Obes Surg. 2023;33(3):743–9.
Li R, Wang K, Qu C, et al. The effect of the enhanced recovery after surgery program on lung cancer surgery: a systematic review and meta-analysis. J Thorac Dis. 2021;13(6):3566–86.
Williams SB, Cumberbatch MGK, Kamat AM, et al. Reporting radical cystectomy outcomes following implementation of enhanced recovery after surgery protocols: a systematic review and individual patient data meta-analysis. Eur Urol. 2020;78(5):719–30.
Ni X, Jia D, Chen Y, et al. Is the Enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J Gastrointest Surg. 2019;23(7):1502–12.
Petrucciani N, Boru CE, Lauteri G, et al. A narrative review on bariatric ERAS. Chirurgia (Bucur). 2022;117(5):505–16.
Papasavas P, Seip RL, McLaughlin T, et al. A randomized controlled trial of an enhanced recovery after surgery protocol in patients undergoing laparoscopic sleeve gastrectomy. Surg Endosc. 2023;37(2):921–31.
Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100(4):482–9.
Geubbels N, Evren I, Acherman YIZ, et al. Randomized clinical trial of an enhanced recovery after surgery programme versus conventional care in laparoscopic Roux-en-Y gastric bypass surgery. BJS Open. 2019;3(3):274–81.
Stenberg E, dos Reis Falcão LF, O’Kane M, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations: a 2021 update. World J Surg. 2022;46(4):729–51.
Ahmed OS, Rogers AC, Bolger JC, et al. Meta-analysis of enhanced recovery protocols in bariatric surgery. J Gastrointest Surg. 2018;22(6):964–72.
Gao B, Chen J, Liu Y, et al. Efficacy and safety of enhanced recovery after surgery protocol on minimally invasive bariatric surgery: a meta-analysis. Int J Surg. 2023;109(4):1015–28.
Małczak P, Pisarska M, Piotr M, et al. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. 2017;27(1):226–35.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Richardson WS, Wilson MC, Nishikawa J, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–3.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3): e088.
Kim H-Y. Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor Dent Endod. 2017;42(2):152–5.
Zhou J, Du R, Wang L, et al. The application of enhanced recovery after surgery (ERAS) for patients undergoing bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2021;31(3):1321–31.
Sierżantowicz R, Ładny JR, Lewko J. Quality of life after bariatric surgery-a systematic review. Int J Environ Res Public Health. 2022;19(15):9078.
Weindelmayer J, Mengardo V, Gasparini A, et al. Enhanced recovery after surgery can improve patient outcomes and reduce hospital cost of gastrectomy for cancer in the west: a propensity-score-based analysis. Ann Surg Oncol. 2021;28(12):7087–94.
Khorgami Z, Petrosky JA, Andalib A, et al. Fast track bariatric surgery: safety of discharge on the first postoperative day after bariatric surgery. Surg Obes Related Dis. 2017;13(2):273–80.
Noba L, Rodgers S, Chandler C, et al. Enhanced recovery after surgery (ERAS) reduces hospital costs and improve clinical outcomes in liver surgery: a systematic review and meta-analysis. J Gastrointest Surg. 2020;24(4):918–32.
Hajibandeh S, Hajibandeh S, Bill V, et al. Meta-analysis of enhanced recovery after surgery (ERAS) protocols in emergency abdominal surgery. World J Surg. 2020;44(5):1336–48.
Olson KA, Fleming RYD, Fox AW, et al. The enhanced recovery after surgery (ERAS) elements that most greatly impact length of stay and readmission. Am Surg. 2021;87(3):473–9.
Pompilio CE, Pelosi P, Castro MG. The bariatric patient in the intensive care unit: pitfalls and management. Curr Atheroscler Rep. 2016;18(9):55.
Shreffler J, Huecker MR. Type I and type II errors and statistical power. In: StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Martin Huecker declares no relevant financial relationships with ineligible companies. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC; 2023.
Higueras A, Gonzalez G, de Lourdes Bolaños M, et al. Economic impact of the implementation of an enhanced recovery after Surgery (ERAS) protocol in a bariatric patient undergoing a Roux-En-Y gastric bypass. Int J Environ Res Public Health. 2022;19(22):14946.
Caiazzo R, Baud G, Clément G, et al. Impact of centralized management of bariatric surgery complications on 90-day mortality. Ann Surg. 2018;268(5):831–7.
Birkmeyer JD. Progress and challenges in improving surgical outcomes. Br J Surg. 2012;99(11):1467–9.
Omar I, Madhok B, Parmar C, et al. Analysis of national bariatric surgery related clinical incidents: lessons learned and a proposed safety checklist for bariatric surgery. Obes Surg. 2021;31(6):2729–42.
Solheim O. Randomized controlled trials in surgery and the glass ceiling effect. Acta Neurochir. 2019;161(4):623–5.
Funding
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• ERAS reduced postoperative nausea and vomiting, intraoperative time, and time to mobilisation following bariatric surgery.
• ERAS also reduced ICU, total hospital, and functional hospital stay times following bariatric surgery.
• Therefore, this study suggests that ERAS should be implemented, where feasible, in all bariatric surgery units.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davey, M.G., Donlon, N.E., Fearon, N.M. et al. Evaluating the Impact of Enhanced Recovery After Surgery Protocols on Surgical Outcomes Following Bariatric Surgery—A Systematic Review and Meta-analysis of Randomised Clinical Trials. OBES SURG 34, 778–789 (2024). https://doi.org/10.1007/s11695-024-07072-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07072-0