Abstract
Purpose
Sleeve gastrectomy with transit bipartition (SG-TB) could be an attractive alternative to Roux-en-Y gastric bypass (RYGB) on weight loss and improvement of comorbidities in patients with obesity. However, there is little long-term data. Translational research on a rat model could allow long-term projection to assess efficacy and safety of SG-TB. The aim of this research was to evaluate the long-term efficacy and safety of SG-TB compared to RYGB and SHAM in rat model.
Materials and Methods
Ninety-four male obese Wistar rats were distributed into 3 groups: SG-TB (n = 34), RYGB (n = 32), and SHAM (control group, n = 28). The percentage of total weight loss (%TWL), coprocalorimetry, glucose and insulin tolerance test, insulin, GLP-1, PYY, and GIP before and after surgery were assessed. The animals were followed over 6 months (equivalent to 16 years in humans).
Results
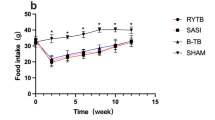
At 6 months, %TWL was significantly greater(p = 0.025) in the SG-TB group compared to the RYGB group. There was no difference between the groups (p = 0.86) in malabsorption 15 and 120 days postoperatively. Glucose tolerance was significantly improved (p = 0.03) in the SG-TB and RYGB groups compared to the preoperative state. Insulin secretion, at 3 months, was significantly more important in the SG-TB group (p = 0.0003), compared to the RYGB and SHAM groups. GLP-1 secretion was significantly increased in the SG-TB and RYGB groups compared to the preoperative state (p = 0.001) but similar between SG-TB and RYGB animals (p = 0.72).
Conclusion
In a rat model, at long term compared to RYGB, SG-TB provides greater and better-maintained weight loss and an increased insulin secretion without impairing nutritional status.
Graphical Abstract







Similar content being viewed by others
References
Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–29.
Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62.
Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297.
Clapp B, Ponce J, DeMaria E, Ghanem O, Hutter M, Kothari S, et al. American Society for Metabolic and Bariatric Surgery 2020 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2022;18:1134–40.
Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI > or =50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244:611–9.
Santoro S, Castro LC, Velhote MCP, Malzoni CE, Klajner S, Castro LP, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256:104–10.
Sarkis R, Khazzaka A, Kassir R. Pilot study of a new model of bariatric surgery: laparoscopic intestinal bipartition-safety and efficacy against metabolic disorders. Obes Surg. 2018;28:3717–23.
Gagner M. Safety and efficacy of a side-to-side duodeno-ileal anastomosis for weight loss and type-2 diabetes: duodenal bipartition, a novel metabolic surgery procedure. Ann Surg Innov Res. 2015;9:6.
Yormaz S, Yılmaz H, Ece I, Sahin M. Laparoscopic ileal interposition with diverted sleeve gastrectomy versus laparoscopic transit bipartition with sleeve gastrectomy for better glycemic outcomes in T2DM patients. Obes Surg. 2018;28:77–86.
Santoro S, Mota FC, Aquino CG. Treating severe GERD and obesity with a sleeve gastrectomy with cardioplication and a transit bipartition. Obes Surg. 2019;29:1439–41.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4:624–30.
Andreollo NA, dos Santos EF, Araújo MR, Lopes LR. Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig. 2012;25(1):49–51.
Tessier R, Ribeiro-Parenti L, Bruneau O, Khodorova N, Cavin J-B, Bado A, et al. Effect of different bariatric surgeries on dietary protein bioavailability in rats. Am J Physiol Gastrointest Liver Physiol. 2019;317:G592–601.
Liu P, Widjaja J, Dolo PR, Yao L, Hong J, Shao Y, et al. Comparing the anti-diabetic effect of sleeve gastrectomy with transit bipartition against sleeve gastrectomy and Roux-en-Y gastric bypass using a diabetic rodent model. Obes Surg. 2021;31:2203–10.
Wang M, Widjaja J, Dolo PR, Yao L, Hong J, Zhu X. The protective effect of transit bipartition and its modification against sleeve gastrectomy-related esophagitis in a rodent model. Obes Surg. 2022;32:1149–56.
Cavin J-B, Voitellier E, Cluzeaud F, Kapel N, Marmuse J-P, Chevallier J-M, et al. Malabsorption and intestinal adaptation after one anastomosis gastric bypass compared with Roux-en-Y gastric bypass in rats. Am J Physiol Gastrointest Liver Physiol. 2016;311:G492–500.
Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014:CD003641. https://doi.org/10.1002/14651858.CD003641.
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(1–190):215–357.
Guimarães M, Osório C, Silva D, Almeida RF, Reis A, Cardoso S, et al. How sustained is Roux-en-Y gastric bypass long-term efficacy?: Roux-en-Y gastric bypass efficacy. Obes Surg. 2021;31:3623–9.
Soong T-C, Lee M-H, Lee W-J, Almalki OM, Chen J-C, Wu C-C, et al. Long-term efficacy of bariatric surgery for the treatment of super-obesity: comparison of SG, RYGB, and OAGB. Obes Surg. 2021;31:3391–9.
Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS, Weidenbacher HJ, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151:1046–55.
Calisir A, Ece I, Yilmaz H, Alptekin H, Acar F, Yormaz S, et al. The mid-term effects of transit bipartition with sleeve gastrectomy on glycemic control, weight loss, and nutritional status in patients with type 2 diabetes mellitus: a retrospective analysis of a 3-year follow-up. Obes Surg. 2021;31:4724–33.
Karaca FC. Effects of sleeve gastrectomy with transit bipartition on glycemic variables, lipid profile, liver enzymes, and nutritional status in type 2 diabetes mellitus patients. Obes Surg. 2020;30:1437–45.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397:293–304.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–51.
Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–54.
Smith EP, Polanco G, Yaqub A, Salehi M. Altered glucose metabolism after bariatric surgery: what’s GLP-1 got to do with it? Metabolism. 2018;83:159–66.
Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–95.
Thondam SK, Cuthbertson DJ, Wilding JPH. The influence of glucose-dependent insulinotropic polypeptide (GIP) on human adipose tissue and fat metabolism: implications for obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Peptides. 2020;125:170208.
Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne). 2021;12:585887.
Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017;36:861–8.
Alvarez-Sola G, Uriarte I, Latasa MU, Fernandez-Barrena MG, Urtasun R, Elizalde M, et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66:1818–28.
Hosseinzadeh A, Roever L, Alizadeh S. Surgery-induced weight loss and changes in hormonally active fibroblast growth factors: a systematic review and meta-analysis. Obes Surg. 2020;30:4046–60.
Rebelos E, Moriconi D, Honka M-J, Anselmino M, Nannipieri M. Decreased weight loss following bariatric surgery in patients with type 2 diabetes. Obes Surg. 2022;33(1):179–87.
Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg. 2020;271:201–9.
Acknowledgements
We thank the group of Prof. N. Kapel of the Department of Functional Coprology, APHP, and especially Dr. L. Barbot-Trystram for stool analyses. We thank N. Sorhaindo, Biochemistry Platform CRI, for plasma samples analyses and Dr. G. Rucher from the Fédération de Recherche en Imagerie Multimodalités (FRIM) for Tomodensitometry analyses.
Funding
Société Française et Francophone de Chirurgie de l’Obésité et des Maladies Métaboliques (SOFFCO-MM), Société francophone de nutrition clinique et métabolisme (SFNCM), AP-HP
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All animal studies comply with the ARRIVE guidelines. They were conducted in accordance with EU directive 2010/63/EU for animal experiments and approved by the Institutional Animal Care and Use Committee (CEA N° 121) and the French Ministry of Higher Education.
Informed Consent
Informed consent does not apply.
Conflict of Interest
Claire Carette reported personal fees from Ipsen Pharma, Pfizer, AstraZeneca, Novo Nordisk, Lilly, Vitalaire, and Merck France outside the submitted work. Sebastien Czernichow reported participation fees from Mygoodlife; personal fees from Novonordisk, Fresenius, Lilly, Janssen, and Bristol Myers Squibb; and other fees from Jellynov outside the submitted work. Tigran Poghosyan reported receiving personal fees from BariaTek, Novo Nordisk, and Gore outside the submitted work. No other disclosures were reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
-In rat, compared to RYGB, SG-TB provides greater and better maintained weight loss.
-Insulin secretion is enhanced in the SG-TB rat model, compared to RYGB.
-In the long term, SG-TB appears to be a sustainable alternative to RYGB.
Maude Le Gall and Tigran Poghosyan are co-last authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baratte, C., Willemetz, A., Ribeiro-Parenti, L. et al. Analysis of the Efficacy and the Long-term Metabolic and Nutritional Status of Sleeve Gastrectomy with Transit Bipartition Compared to Roux-en-Y Gastric Bypass in Obese Rats. OBES SURG 33, 1121–1132 (2023). https://doi.org/10.1007/s11695-023-06477-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06477-7