Abstract
Background
Morbidly obese patients (BMI > 40 kg/m2) are at increased risk for venous thromboembolism, especially after surgery. Despite limited evidence, morbidly obese patients are often administered a double dose of nadroparin for thromboprophylaxis compared to non-obese patients. The aim of this study was to evaluate the influence of different body size descriptors on anti-Xa levels after a double dose of nadroparin (5,700 IU) in morbidly obese patients.
Methods
In 27 morbidly obese patients with a mean total body weight of 148 kg (range 107–260 kg), anti-Xa levels were determined peri-operatively until 24 h after administration of a subcutaneous dose of 5,700 IU of nadroparin.
Results
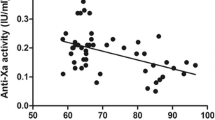
Anti-Xa level 4 h after administration (A4h, mean 0.22 ± 0.07 IU/ml) negatively correlated strongly with lean body weight (r = −0.66 (p < 0.001)) and moderately with total body weight (r = −0.56 (p = 0.003)) and did not correlate with body mass index (r = −0.26 (p = 0.187)). The area under the anti-Xa level-time curve from 0 to 24 h (AUA0–24h, mean 2.80 ± 0.97 h IU/ml) correlated with lean body weight (r = −0.63 (p = 0.007)), but did not correlate with total body weight (r = −0.44 (p = 0.075)) or body mass index (r = −0.10 (p = 0.709)).
Conclucions
Following a subcutaneous dose of nadroparin 5,700 IU, A4h and AUA0–24h were found to negatively correlate strongly with lean body weight. From these results, individualized dosing of nadroparin based on lean body weight should be considered in morbidly obese patients.



Similar content being viewed by others
References
Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–55.
Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118(9):978–80.
Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887–90.
Duplaga BA, Rivers CW, Nutescu E. Dosing and monitoring of low-molecular-weight heparins in special populations. Pharmacotherapy. 2001;21(2):218–34.
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S–453S.
Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188S–203S.
Nutescu EA, Spinler SA, Wittkowsky A, et al. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–83.
Rondina MT, Wheeler M, Rodgers GM, et al. Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-ill patients. Thromb Res. 2010;125(3):220–3.
Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4(5):625–31.
Singh K, Podolsky ER, Um S, et al. Evaluating the safety and efficacy of BMI-based preoperative administration of low-molecular-weight heparin in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2011 Apr 9. doi:10.1007/s11695-011-0397-y.
Kalfarentzos F, Stavropoulou F, Yarmenitis S, et al. Prophylaxis of venous thromboembolism using two different doses of low-molecular-weight heparin (nadroparin) in bariatric surgery: a prospective randomized trial. Obes Surg. 2001;11(6):670–6.
Janmahasatian S, Duffull SB, Ash S, et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Rowland M, Tozer T. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2010.
Agnelli G, Iorio A, Renga C, et al. Prolonged antithrombin activity of low-molecular-weight heparins. Clinical implications for the treatment of thromboembolic diseases. Circulation. 1995;92(10):2819–24.
Boneu B, Navarro C, Cambus JP, et al. Pharmacodynamics and tolerance of two nadroparin formulations (10,250 and 20,500 anti Xa IU x ml(-1)) delivered for 10 days at therapeutic dose. Thromb Haemost. 1998;79(2):338–41.
Collignon F, Frydman A, Caplain H, et al. Comparison of the pharmacokinetic profiles of three low molecular mass heparins—dalteparin, enoxaparin and nadroparin—administered subcutaneously in healthy volunteers (doses for prevention of thromboembolism). Thromb Haemost. 1995;73(4):630–40.
Freedman MD, Leese P, Prasad R, et al. An evaluation of the biological response to fraxiparine, (a low molecular weight heparin) in the healthy individual. J Clin Pharmacol. 1990;30(8):720–7.
Harenberg J, Wurzner B, Zimmermann R, et al. Bioavailability and antagonization of the low molecular weight heparin CY 216 in man. Thromb Res. 1986;44(4):549–54.
Heizmann M, Baerlocher GM, Steinmann F, et al. Anti-Xa activity in obese patients after double standard dose of nadroparin for prophylaxis. Thromb Res. 2002;106(4–5):179–81.
Harenberg J. Is laboratory monitoring of low-molecular-weight heparin therapy necessary? Yes. J Thromb Haemost. 2004;2(4):547–50.
Paige JT, Gouda BP, Gaitor-Stampley V, et al. No correlation between anti-factor Xa levels, low-molecular-weight heparin, and bleeding after gastric bypass. Surg Obes Relat Dis. 2007;3(4):469–75.
Bounameaux H, de Moerloose P. Is laboratory monitoring of low-molecular-weight heparin therapy necessary? No. J Thromb Haemost. 2004;2(4):551–4.
Barras MA, Duffull SB, Atherton JJ, et al. Individualized compared with conventional dosing of enoxaparin. Clin Pharmacol Ther. 2008;83(6):882–8.
Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6.
Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26 Suppl 2:24–38.
Sanderink GJ, Le Liboux A, Jariwala N, et al. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72(3):308–18.
Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26(4):610–5.
Acknowledgements
The authors would like to thank Brigitte Bliemer and Silvia Samson for their enthusiastic support and participation in this study. Esther Janssen is acknowledged for her contribution to this study and Saskia de Mik-van Ham for the editorial assistance.
Conflict of interest
There is no conflict of interest for all authors: Jeroen Diepstraten, Christian M. Hackeng, Simone van Kralingen, Jiri Zapletal, Eric P.A. van Dongen, René J. Wiezer, Bert van Ramshorst, Catherijne A.J. Knibbe
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diepstraten, J., Hackeng, C.M., van Kralingen, S. et al. Anti-Xa Levels 4 h After Subcutaneous Administration of 5,700 IU Nadroparin Strongly Correlate with Lean Body Weight in Morbidly Obese Patients. OBES SURG 22, 791–796 (2012). https://doi.org/10.1007/s11695-012-0602-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0602-7