Abstract
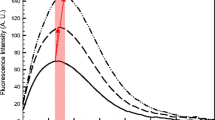
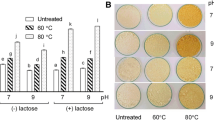
This study investigated the structural and functional changes of the interaction between preheated whey protein isolate (WPI) and anthocyanin (AN) at different temperatures through Fourier transform infrared spectroscopy, endogenous fluorescence spectroscopy, and foaming properties. The results showed that preheat treatment reduced the average particle size of the solution and increased the absolute value of ζ-potential, and the whey protein isolate after heat treatment at 70 °C had the highest binding rate to anthocyanins, which increased from 87.44 to 89.67%. Preheat treatment significantly increased the surface hydrophobicity of WPI, but anthocyanins decreased the surface hydrophobicity of the composites. Fourier transform infrared spectroscopy results indicated that preheat treatment changed the secondary structure of the WPI and WPI-AN complexes, with the α-helix content decreasing and the β-fold content increasing. The endogenous fluorescence spectra analysis illustrated the preheating treatment exposed the internal chromogenic groups of the protein and that anthocyanins increased the polarity of the microenvironment of the protein fluorescent groups and changed the conformation. The foaming properties results revealed that the combination of AN improved the foamability and foam stability of WPI, and the foaming properties of the protein was significantly improved within a certain heat treatment temperature range. In this study, preheat treatment and complexation of anthocyanins were used to improve the structural and functional properties of whey isolate protein in expectation of improving the utilization value of WPI and to provide motivation and data reference for further application of WPI in food industry.
Highlights
1. The preheated WPI contributes to the formation of WPI-AN composites.
2. The addition of anthocyanins led to changes in the structure and properties of preheated WPI.
3. The combination of AN improved the foamability and foam stability of WPI, and the foaming properties of the protein was significantly improved within a certain heat treatment temperature range.






Similar content being viewed by others
References
A. Kilara, M.N. Vaghela. Whey proteins - sciencedirect. Proteins in Food Processing (Second Edition), 93–126 (2018). https://doi.org/10.1016/B978-0-08-100722-8.00005-X
Patel, Seema, Functional food relevance of whey protein: a review of recent findings and scopes ahead. J. Funct. Foods 19, 308–319 (2015). https://doi.org/10.1016/j.jff.2015.09.040
W. Chen, W. Wang, X. Ma, R. Lv, R.B. Watharkar, T. Ding, et al, Effect of ph-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (-)-epigallocatechin-3-gallate. Food Chem 274(FEB.15), 234–241 (2019). https://doi.org/10.1016/j.foodchem.2018.08.106
Y. Hu, G. Kou, Q. Chen, Y. Li, Z. Zhou, Protection and delivery of mandarin (citrus reticulata blanco) peel extracts by encapsulation of whey protein concentrate nanoparticles. Lebensmittel Wissenschaft Und Technologie 99, 24–33 (2019). https://doi.org/10.1016/j.lwt.2018.09.044
O. Gulay, I. Paola, Franco, D. Marco et al. A review of microencapsulation methods for food antioxidants: principles, advantages, drawbacks and applications. Food chemistry. (2018). https://doi.org/10.1016/j.foodchem.2018.07.205
C. Jaroslaw, Krzysztof, & Dwiecki. (2017). A review of methods used for investigation of protein–phenolic compound interactions. International Journal of Food Science & Technology. (2017) https://doi.org/10.1111/ijfs.13339
T.H. Quan, S. Benjakul, T. Sae-Leaw, A.K. Balange, S. Maqsood. Protein–polyphenol conjugates: antioxidant property, functionalities and their applications. Trends Food Sci. Technol, 91(2019). https://doi.org/10.1016/j.tifs.2019.07.049
X. Sui, H. Sun, B. Qi, M. Zhang, Y. Li, L. Jiang, Functional and conformational changes to soy proteins accompanying anthocyanins: focus on covalent and non-covalent interactions. Food Chem 245(apr.15), 871–878 (2017). https://doi.org/10.1016/j.foodchem.2017.11.090
Jakobek, Lidija, Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem 175, 556–567 (2015). https://doi.org/10.1016/j.foodchem.2014.12.013
W. Chen, T. Li, H. Yu, C. Ma, X. Wang, A. Qayum, et al, Structure and Emulsifying Properties of whey Protein Isolate: Effect of Safflower Yellow concentration - sciencedirect (LWT, 2020), p. 123, https://doi.org/10.1016/j.lwt.2020.109079
G. Chen, S. Wang, B. Feng, B. Jiang, M. Miao. Interaction between soybean protein and tea polyphenols under high pressure. Food Chemistry, 277(MAR.30), 632–638 (2019). https://doi.org/10.1016/j.foodchem.2018.11.024
Z. Zang, S. Chou, L. Geng, X. Si, Y. Ding, Y. Lang, et al. (2021) .Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: Color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT, 152(2021). https://doi.org/10.1016/j.lwt.2021.112269
A. Smeriglio, D. Barreca, E. Bellocco, D. Trombetta, Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res (2016). https://doi.org/10.1002/ptr.5642
J. Ge, P. Yue, J. Chi, J. Liang, X. Gao, Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll 74, 23–31 (2018). https://doi.org/10.1016/j.foodhyd.2017.07.029
P.N. Chen, S.C. Chu, H.L. Chiou, W.H. Kuo, C.L. Chiang, Y.S. Hsieh, Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett 235(2), 248–259 (2006). https://doi.org/10.1016/j.canlet.2005.04.033
Z. Zang, S. Chou, J. Tian, Y. Lang, B. Li. (2020). Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: a mechanistic and in vitro simulation study. Food Chemistry, 336, 127700 (2020). https://doi.org/10.1016/j.foodchem.2020.127700
R.O. Sinnhuber, T.C. Yu, The 2-thiobarbituric acid reaction, an objective measure of the oxidative deterioration occurring in fats and oils. J. Japan Oil Chemists\“ Soc. 26(5), 259–267 (1977)
H.M. Rawel, K. Meidtner, J. Kroll. (2005). Binding of selected phenolic compounds to proteins. Journal of Agricultural & Food Chemistry, 53(10), 4228 (2005). https://doi.org/10.1021/jf0480290
Z.L. Wan, J.M. Wang, L.Y. Wang, Y. Yang, X.Q. Yang, Complexation of resveratrol with soy protein and its improvement on oxidative stability of corn oil/water emulsions. Food Chem 161(oct.15), 324–331 (2014). https://doi.org/10.1016/j.foodchem.2014.04.028
E. Luis, E. Rodriguez-Saona, Ronald, & Wrolstad. Extraction, isolation, and purification of anthocyanins. Curr. Protocols Food Anal. Chem. (2001). https://doi.org/10.1002/0471142913.faf0101s00
X.N. Sui, X. Dong, W.B. Zhou, Combined effect of ph and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. (2017). https://doi.org/10.1007/978-981-10-2612-6_4
E. Sadilova, F.C. Stintzing, R. Carle, Thermal degradation of Acylated and Nonacylated Anthocyanins[J]. J. Food Sci 71(8), C504–C512 (2010)
M.K. Kim, H. Kim, K. Koh, H.S. Kim, Y.S. Lee, Y.H. Kim, Identification and quantification of anthocyanin pigments in colored rice. Nutr. Res. Pract. 2(1), 46–49 (2008)
A.M. Herrero, P. Carmona, T. Pintado, F. Jiménez-Colmenero, & C Ruíz-Capillas. (2011). Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Research International, 44(1), 360–366 (2011). https://doi.org/10.1016/j.foodres.2010.10.006
F.J. Morales, S. Jiménez-Pérez. (2001). Free radical scavenging capacity of maillard reaction products as related to color and fluorescence. Food Chemistry, 72(1), 119–125 (2001). https://doi.org/10.1016/S0308-8146(00)00239-9
B.I. Kurganov (2002). Kinetics of protein aggregation. quantitative estimation of the chaperone-like activity in test-systems based on suppression of protein aggregation. Biochemistry, 67(4), 409–422 (2002). https://doi.org/10.1023/A:1015277805345
C.A. Haskard, E.C.Y. Li-Chan, Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (prodan) and anionic (ans-) fluorescent probes. J. Agricultural Food Chem. 46(7), 2671–2677 (1998)
M. Naczk, S. Grant, R. Zadernowski, E. Barre, Protein precipitating capacity of phenolics of wild blueberry leaves and fruits. Food Chem 96(4), 640–647 (2006). https://doi.org/10.1016/j.foodchem.2005.03.017
H. Bouaouina, A. Desrumaux, C. Loisel, J. Legrand, Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int. Dairy J 16(4), 275–284 (2006). https://doi.org/10.1016/j.idairyj.2005.05.004
P. Chanphai, P. Bourassa, C.D. Kanakis, P.A. Tarantilis, M.G. Polissiou, H.A. Tajmir-Riahi. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocolloids, 77(APR.), 322–328 (2018). https://doi.org/10.1016/j.foodhyd.2017.10.008
G. Unterhaslberger, C. Schmitt, C. Sanchez, C. Appolonia-Nouzille, A. Raemy, Heat denaturation and aggregation of β-lactoglobulin enriched wpi in the presence of arginine hcl, nacl and guanidinium hcl at ph 4.0 and 7.0. Food Hydrocoll 20(7), 1006–1019 (2006)
S.M. Fitzsimons, D.M. Mulvihill, E.R. Morris, Denaturation and aggregation processes in thermal gelation of whey proteins resolved by differential scanning calorimetry. Food Hydrocoll 21(4), 638–644 (2007). https://doi.org/10.1016/j.foodhyd.2006.07.007
H.J. Kim, E.A. Decker, D.J. Mcclements, Role of postadsorption conformation changes of β-lactoglobulin on its ability to stabilize oil droplets against flocculation during heating at neutral ph. Langmuir 18(20), 7577–7583 (2002). https://doi.org/10.1021/la020385u
E. Daniela, F.A. Igartúa, A. Platania, G.G. Balcone, Palazolo, M. Dario, Cabezas, Impact of heat treatment in whey proteins-soluble soybean polysaccharides electrostatic complexes in different pH and biopolymer mass ratio conditions. Appl. Food Res. 2(2), 2772–5022 (2022)
D. Jian, Z. Xu, B. Qi, L. Jiang, X. Sui, Physicochemical and oxidative stability of a soybean oleosome-based emulsion and its in vitro digestive fate as affected by -epigallocatechin-3-gallate. Food Funct 9(12), 6146–6154 (2018)
Z. Wei, Y. Wei, F. Rui, Y. Fang, Y. Gao, Evaluation of structural and functional properties of protein–egcg complexes and their ability of stabilizing a model β-carotene emulsion. Food Hydrocoll 45(mar.), 337–350 (2015)
Q.T. Zhang, Z.C. Tu, H. Xiao, H. Wang, X.Q. Huang, G.X. Liu, et al, Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food & Bioproducts Processing 92(1), 30–37 (2014)
Y. Zhang, Q. Chen, Si, Baokun et al., Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int (2018). https://doi.org/10.1016/j.foodchem.2018.07.170
L. Jian, Y. Liu, L. Li, B. Qi, M. Ju, Y. Xu, et al, Covalent conjugates of anthocyanins to soy protein: unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int 120(JUN.), 603–609 (2019)
X. Zhao, F. Chen, W. Xue, L. Lee, Ftir spectra studies on the secondary structures of 7s and 11s globulins from soybean proteins using aot reverse micellar extraction. Food Hydrocoll 22(4), 568–575 (2008)
Y. Moriyama, Y. Kawasaka, K. Takeda, Protective effect of small amounts of sodium dodecyl sulfate on the helical structure of bovine serum albumin in thermal denaturation. J. Colloid Interface Sci 257(1), 41–46 (2003)
Z. Chen, C. Wang, X. Gao, Y. Chen, R.K. Santhanam, C. Wang, et al, Interaction characterization of preheated soy protein isolate with cyanidin-3-o-glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts - sciencedirect. Food Chem 271, 266–273 (2019)
J. Jiang, Z. Zhang, Z. Jing, Y. Liu, The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem 268, 334–341 (2018)
Y. Zhang, Q. Zhong, Binding between bixin and whey protein at ph 7.4 studied by spectroscopy and isothermal titration calorimetry. J. Agric. Food Chem. 60(7), 1880–1886 (2012)
J. Wei, D. Xu, J. Yang, X. Zhang, T. Mu, Q. Wang, Analysis of the interaction mechanism of anthocyanins (aronia melanocarpa elliot) with β-casein. Food Hydrocoll 84(NOV.), 276–281 (2018)
O.G. Jones, D.J. Mcclements, Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complexes. Adv. Colloid Interface Sci 167(1–2), 49–62 (2011)
J.K. Chan, T.A. Gill, Thermal aggregation of mixed fish myosins. J. Agric. Food Chem 42(12), 2649–2655 (1994)
X. Wang, Z. Jiao, F. Lei, C. Liang, F. Yuan, Y. Gao, Covalent complexation and functional evaluation of (–)-epigallocatechin gallate and α-lactalbumin. Food Chem 150(may 1), 341–347 (2014)
J. Kroll, H.M. Rawel, N. Seidelmann, Physicochemical properties and susceptibility to proteolytic digestion of myoglobin-phenol derivatives. J. Agricultural Food Chem. 48(5), 1580 (2002). https://doi.org/10.1021/jf991172m
H.M. Rawel, S. Rohn, H.P. Kruse, Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem 78(4), 443–455 (2002). https://doi.org/10.1016/S0308-8146(02)00155-3
H.M. Rawel, D. Czajka, S. Rohn, J. Kroll, Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol 30(3–4), 137–150 (2002). https://doi.org/10.1016/S0141-8130(02)00016-8
N. Gharbi, M. Labbafi, Influence of treatment-induced modification of egg white proteins on foaming properties. Food Hydrocoll 90(MAY), 72–81 (2019). https://doi.org/10.1016/j.foodhyd.2018.11.060
Y. Meng, C. Li, Conformational changes and functional properties of whey protein isolate-polyphenol complexes formed by non-covalent interaction. Food Chem 364(1), 129622 (2021). https://doi.org/10.1016/j.foodchem.2021.129622
Acknowledgements
This work has been funded by the National Soybean Industrial Technology System of China (CARS-04-PS32) and Heilongjiang Province Key S&T Program (2019ZX08B01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Declaration of competing interest
All the authors declare that he/she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Z., Cui, Y., Zhang, A. et al. The effect of preheated WPI interaction with AN on its complexes based on protein structure and function. Food Measure 17, 3272–3282 (2023). https://doi.org/10.1007/s11694-023-01867-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01867-y