Abstract
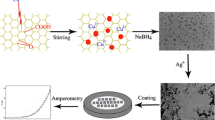
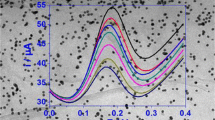
In this work, The production of graphene nanoribbon (GNR)/CoFe2O4 nanocomposite is described using a simple, cost-effective, and innovative technique. X-ray diffraction (XRD), transmission electron microscopy (TEM), and field emission scanning electron microscopy (FESEM) were proved that graphene nanoribbons and GNR-CoFe2O4 nanocomposite were successfully synthesized. The designed nano-sensor was employed for the determination of vanillin using a carbon paste electrode (CPE) modified with graphene nanoribbons-CoFe2O4 nanocomposite and 1-methyl-3-butylimidazolium bromide (ionic liquid (IL)). The electrochemical behavior modified electrode for vanillin detection exhibited excellent electrocatalytic activity with a high peak current. Under the optimized conditions, the calibration curve offered linearity over a wide range of 0.01–500 µM, with a limit of detection of 5.2 nM. The fabricated nanosensor was successfully utilized for determination of vanillin in commercial real food samples with an outstanding recovery.








Similar content being viewed by others
References
P. Teissedre, A. Waterhouse, Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J. Agric. Food Chem. 48(9), 3801–3805 (2000)
P. Deng, Z. Xu, R. Zeng, C. Ding, Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 180, 156–163 (2015)
T.R. Silva, D. Brondani, E. Zapp, I. Cruz Vieira, Electrochemical sensor based on gold nanoparticles stabilized in poly (allylamine hydrochloride) for determination of vanillin. Electroanalysis 27(2), 465–472 (2015)
L. Jiang, Y. Ding, F. Jiang, L. Li, F. Mo, Electrodeposited nitrogen-doped graphene/carbon nanotubes nanocomposite as enhancer for simultaneous and sensitive voltammetric determination of caffeine and vanillin. Anal. Chim. Acta 833, 22–28 (2014)
W. Wu, L. Yang, F. Zhao, B. Zeng, A vanillin electrochemical sensor based on molecularly imprinted poly (1-vinyl-3-octylimidazole hexafluoride phosphorus) – multi-walled carbon nanotubes@ polydopamine–carboxyl single-walled carbon nanotubes composite. Sens. Actuators B 239, 481–487 (2017)
A. Cifuentes, Advanced separation methods in food analysis. J. Chromatogr. A 1216(43), 7109–7358 (2009)
T. Zabihpour, S.-A. Shahidi, H. Karimi-Maleh, A. Ghorbani-HasanSaraei, Voltammetric food analytical sensor for determining vanillin based on amplified NiFe 2 O 4 nanoparticle/ionic liquid sensor. J. Food Meas. Charact. (2020). https://doi.org/10.1007/s11694-019-00353-8
D. Yang, Y. Ying, Applications of Raman spectroscopy in agricultural products and food analysis: A review. Appl. Spectrosc. Rev. 46(7), 539–560 (2011)
H. Jin, Q. Lu, X. Chen, H. Ding, H. Gao, S. Jin, The use of Raman spectroscopy in food processes: A review. Appl. Spectrosc. Rev. 51(1), 12–22 (2016)
S. Altınöz, S. Toptan, Simultaneous determination of Indigotin and Ponceau-4R in food samples by using Vierordt’s method, ratio spectra first order derivative and derivative UV spectrophotometry. J. Food Compos. Anal. 16(4), 517–530 (2003)
M. Mahboubifar, Z. Sobhani, G. Dehghanzadeh, K. Javidnia, A comparison between UV spectrophotometer and high-performance liquid chromatography method for the analysis of sodium benzoate and potassium sorbate in food products. Food Anal. Methods 4(2), 150–154 (2011)
O.A. Adebo, S.A. Oyeyinka, J.A. Adebiyi, X. Feng, J.D. Wilkin, Y.O. Kewuyemi, A.M. Abrahams, F. Tugizimana, Application of gas chromatography–mass spectrometry (GC-MS)‐based metabolomics for the study of fermented cereal and legume foods: A review. Int. J. Food Sci. Technol. 56(4), 1514–1534 (2021)
W. Yan-Wen, L. Bing-Ning, L. Ling-Ling, Research progress of analysis of mineral oil hydrocarbons using on-line high performance liquid chromatography coupled with gas chromatography. Chin. J. Anal. Chem. 49(3), 341–349 (2021)
J. Lindeberg, Capillary electrophoresis in food analysis. Food Chem. 55(1), 73–94 (1996)
S. Cheraghi, M.A. Taher, H. Karimi-Maleh, Highly sensitive square wave voltammetric sensor employing CdO/SWCNTs and room temperature ionic liquid for analysis of vanillin and folic acid in food samples. J. Food Compos. Anal. 62, 254–259 (2017)
H. Medetalibeyoğlu, M. Beytur, S. Manap, C. Karaman, F. Kardaş, O. Akyıldırım, G. Kotan, H. Yüksek, N. Atar, M.L. Yola, Molecular imprinted sensor including Au nanoparticles/polyoxometalate/two-dimensional hexagonal boron nitride nanocomposite for diazinon recognition. ECS J. Solid State Sci. Technol. 9(10), 101006 (2020)
C. Karaman, O. Karaman, B.B. Yola, İ Ulker, N. Atar, M.L. Yola, A novel electrochemical Aflatoxin B1 immunosensor based on gold nanoparticles decorated porous graphene nanoribbon and Ag nanocubes incorporated MoS2 nanosheets. New J. Chem. 45, 11222–11233 (2021)
H. Karimi-Maleh, F. Karimi, L. Fu, A.L. Sanati, M. Alizadeh, C. Karaman, Y. Orooji, Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 423, 127058 (2021)
H. Karimi-Maleh, M.L. Yola, N. Atar, Y. Orooji, F. Karimi, P.S. Kumar, J. Rouhi, M. Baghayeri, A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@ MOF-74 nanocomposite. J. Colloid Interface Sci. 592, 174–185 (2021)
H. Karimi-Maleh, Y. Orooji, F. Karimi, M. Alizadeh, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V.K. Gupta, A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 184, 113252 (2021)
G. Ziyatdinova, E. Kozlova, E. Ziganshina, H. Budnikov, Surfactant/carbon nanofibers-modified electrode for the determination of vanillin. Monatsh. Chem. 147(1), 191–200 (2016)
N.K. Swamy, K.N.S. Mohana, M.B. Hegde, A.M. Madhusudana, K. Rajitha, S.R. Nayak, Fabrication of graphene nanoribbon-based enzyme-free electrochemical sensor for the sensitive and selective analysis of rutin in tablets. J. Appl. Electrochem. 51(7), 1047–1057 (2021)
M. Fouladgar, Application of ZnO nanoparticle/ion liquid modified carbon paste electrode for determination of isoproterenol in pharmaceutical and biological samples. J. Electrochem. Soc. 163(3), B38 (2015)
Y. Orooji, P.N. Asrami, H. Beitollahi, S. Tajik, M. Alizadeh, S. Salmanpour, M. Baghayeri, J. Rouhi, A.L. Sanati, F. Karimi, An electrochemical strategy for toxic ractopamine sensing in pork samples; twofold amplified nano-based structure analytical tool. J. Food Meas. Charact. (2021). https://doi.org/10.1007/s11694-021-00982-y
S. Negahban, M. Fouladgar, G. Amiri, Improve the performance of carbon paste electrodes for determination of dobutamine using MnZnFe2O4 nanoparticles and ionic liquid. J. Taiwan Inst. Chem. Eng. 78, 51–55 (2017)
E. Vatandost, A. Ghorbani-HasanSaraei, F. Chekin, S.N. Raeisi, S.-A. Shahidi, Green tea extract assisted green synthesis of reduced graphene oxide: Application for highly sensitive electrochemical detection of sunset yellow in food products. Food Chem. X 6, 100085 (2020)
H. Sadeghi, S.-A. Shahidi, S.N. Raeisi, A. Ghorbani-HasanSaraei, F. Karimi, Electrochemical determination of vitamin B6 in water and juice samples using an electrochemical sensor amplified with NiO/CNTs and Ionic liquid. Int. J. Electrochem. Sci. 15, 10488–10498 (2020)
H. Arzaghi, B. Rahimi, B. Adel, G. Rahimi, Z. Taherian, A.L. Sanati, A.S. Dezfuli, Nanomaterials modulating stem cells behavior towards cardiovascular cell linage. Mater. Adv. (2021). https://doi.org/10.1039/D0MA00957A
F. Karimi, A. Ayati, B. Tanhaei, A.L. Sanati, S. Afshar, A. Kardan, Z. Dabirifar, C. Karaman, Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 203, 111753 (2021)
M. Al Sharabati, R. Abokwiek, A. Al-Othman, M. Tawalbeh, C. Karaman, Y. Orooji, F. Karimi, Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 202, 111694 (2021)
A. Aykan, O. KARAMAN, C. KARAMAN, N. Atar, M.L. Yola, A comparative study of CO catalytic oxidation on the single vacancy and di-vacancy graphene supported single-atom iridium catalysts: A DFT analysis. Surf. Interfaces 25, 101293 (2021)
C. Karaman, O. Karaman, N. Atar, M.L. Yola, Sustainable electrode material for high-energy supercapacitor: biomass-derived graphene-like porous carbon with three-dimensional hierarchically ordered ion highways. Phys. Chem. Chem. Phys. 23(22), 12807–12821 (2021)
C. Karaman, E. Bayram, O. Karaman, Z. Aktaş, Preparation of high surface area nitrogen doped graphene for the assessment of morphologic properties and nitrogen content impacts on supercapacitors. J. Electroanal. Chem. 868, 114197 (2020)
T. Eren, N. Atar, M.L. Yola, H. Karimi-Maleh, A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 185, 430–436 (2015)
A.A. Ensafi, H. Karimi-Maleh, Voltammetric determination of isoproterenol using multiwall carbon nanotubes‐ionic liquid paste electrode. Drug. Test. Anal. 3(5), 325–330 (2011)
A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, N‐(3, 4‐Dihydroxyphenethyl)‐3, 5‐dinitrobenzamide‐Modified Multiwall Carbon Nanotubes Paste Electrode as a Novel Sensor for Simultaneous Determination of Penicillamine, Uric acid, and Tryptophan. Electroanalysis 23(6), 1478–1487 (2011)
A.L. Sanati, F. Faridbod, Electrochemical determination of methyldopa by graphene quantum dot/1-butyl-3-methylimidazolium hexafluoro phosphate nanocomposite electrode. Int. J. Electrochem. Sci. 12, 7997–8005 (2017)
F. Faridbod, A.L. Sanati, Graphene quantum dots in electrochemical sensors/biosensors. Curr. Anal. Chem. 15(2), 103–123 (2019)
A.L. Sanati, F. Faridbod, M.R. Ganjali, Synergic effect of graphene quantum dots and room temperature ionic liquid for the fabrication of highly sensitive voltammetric sensor for levodopa determination in the presence of serotonin. J. Mol. Liq. 241, 316–320 (2017)
J. Raoof, R. Ojani, H. Karimi-Maleh, Electrocatalytic oxidation of glutathione at carbon paste electrode modified with 2, 7-bis (ferrocenyl ethyl) fluoren-9-one: application as a voltammetric sensor. J. Appl. Electrochem. 39(8), 1169–1175 (2009)
A.A. Ensafi, M. Taei, T. Khayamian, H. Karimi-Maleh, F. Hasanpour, Voltammetric measurement of trace amount of glutathione using multiwall carbon nanotubes as a sensor and chlorpromazine as a mediator. J. Solid State Electrochem. 14(8), 1415–1423 (2010)
A.A. Ensafi, E. Khoddami, B. Rezaei, H. Karimi-Maleh, p-Aminophenol–multiwall carbon nanotubes–TiO2 electrode as a sensor for simultaneous determination of penicillamine and uric acid. Colloids Surf., B 81(1), 42–49 (2010)
B.J. Sanghavi, P.K. Kalambate, S.P. Karna, A.K. Srivastava, Voltammetric determination of sumatriptan based on a graphene/gold nanoparticles/Nafion composite modified glassy carbon electrode. Talanta 120, 1–9 (2014)
B.J. Sanghavi, A.K. Srivastava, Simultaneous voltammetric determination of acetaminophen and tramadol using Dowex50wx2 and gold nanoparticles modified glassy carbon paste electrode. Anal. Chim. Acta 706(2), 246–254 (2011)
S. Tajik, Y. Orooji, Z. Ghazanfari, F. Karimi, H. Beitollahi, R.S. Varma, H.W. Jang, M. Shokouhimehr, Nanomaterials modified electrodes for electrochemical detection of Sudan I in food. J. Food Meas. Charact. (2021). https://doi.org/10.1007/s11694-021-00955-1
K.M. Cho, S.-Y. Cho, S. Chong, H.-J. Koh, D.W. Kim, J. Kim, H.-T. Jung, Edge-functionalized graphene nanoribbon chemical sensor: comparison with carbon nanotube and graphene. ACS Appl. Mater. Interfaces 10(49), 42905–42914 (2018)
R. Sainz, M. Del Pozo, M. Vilas-Varela, J. Castro-Esteban, M.P. Corral, L. Vázquez, E. Blanco, D. Peña, J.A. Martín-Gago, G.J. Ellis, Chemically synthesized chevron-like graphene nanoribbons for electrochemical sensors development: determination of epinephrine. Sci. Rep. 10(1), 1–11 (2020)
D.A. Brownson, D.K. Kampouris, C.E. Banks, Graphene electrochemistry: fundamental concepts through to prominent applications. Chem. Soc. Rev. 41(21), 6944–6976 (2012)
Z. Wang, Q. Li, H. Zheng, H. Ren, H. Su, Q. Shi, J. Chen, Tuning the electronic structure of graphene nanoribbons through chemical edge modification: A theoretical study. Phys. Rev. B 75(11), 113406 (2007)
M. Terrones, A.R. Botello-Méndez, J. Campos-Delgado, F. López-Urías, Y.I. Vega-Cantú, F.J. Rodríguez-Macías, A.L. Elías, E. Munoz-Sandoval, A.G. Cano-Márquez, J.-C. Charlier, Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 5(4), 351–372 (2010)
P. Dong, T. Zhang, H. Xiang, X. Xu, Y. Lv, Y. Wang, C. Lu, Controllable synthesis of exceptionally small-sized superparamagnetic magnetite nanoparticles for ultrasensitive MR imaging and angiography. J. Mater. Chem. B 9(4), 958–968 (2021)
L. Zhang, M. Zhang, S. You, D. Ma, J. Zhao, Z. Chen, Effect of Fe3 + on the sludge properties and microbial community structure in a lab-scale A2O process. Sci. Total Environ. 780, 146505 (2021)
Z. Es’ haghzade, E. Pajootan, H. Bahrami, M. Arami, Facile synthesis of Fe3O4 nanoparticles via aqueous based electro chemical route for heterogeneous electro-Fenton removal of azo dyes. J. Taiwan Inst. Chem. Eng. 71, 91–105 (2017)
Z. Ni, X. Cao, X. Wang, S. Zhou, C. Zhang, B. Xu, Y. Ni, Facile synthesis of copper (I) oxide nanochains and the photo-thermal conversion performance of its nanofluids. Coatings 11(7), 749 (2021)
C. Karaman, Boosting effect of nitrogen and phosphorous Co-doped three-dimensional graphene architecture: Highly selective electrocatalysts for carbon dioxide electroreduction to formate. Top. Catal. (2021). https://doi.org/10.1007/s11244-021-01500-6
M.M. Fard, H. Beiki, Experimental investigation of benzoic acid diffusion coefficient in γ-Al 2 O 3 nanofluids at different temperatures. Heat Mass Transf. 52(10), 2203–2211 (2016)
A. Afkhami, F. Gomar, T. Madrakian, CoFe2O4 nanoparticles modified carbon paste electrode for simultaneous detection of oxycodone and codeine in human plasma and urine. Sens. Actuators B 233, 263–271 (2016)
C. Raril, J. Manjunatha, A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode. Microchem. J. 154, 104575 (2020)
J. Peng, C. Hou, X. Hu, A graphene-based electrochemical sensor for sensitive detection of vanillin. Int. J. Electrochem. Sci. 7(2), 1724–1733 (2012)
L. Shang, F. Zhao, B. Zeng, Sensitive voltammetric determination of vanillin with an AuPd nanoparticles – graphene composite modified electrode. Food Chem. 151, 53–57 (2014)
L. Huang, K. Hou, X. Jia, H. Pan, M. Du, Preparation of novel silver nanoplates/graphene composite and their application in vanillin electrochemical detection. Mater. Sci. Eng. C 38, 39–45 (2014)
M.A. Khalilzadeh, Z. Arab, High sensitive nanostructure square wave voltammetric sensor for determination of vanillin in food samples. Curr. Anal. Chem. 13(1), 81–86 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roostaee, M., Sheikhshoaie, I. A novel, sensitive and selective nanosensor based on graphene nanoribbon–cobalt ferrite nanocomposite and 1-methyl-3-butylimidazolium bromide for detection of vanillin in real food samples. Food Measure 16, 523–532 (2022). https://doi.org/10.1007/s11694-021-01180-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01180-6