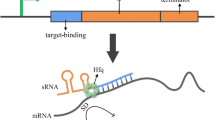
Abstract
Small RNAs (sRNAs) are genetic tools for the efficient and specific tuning of target genes expression in bacteria. Inspired by naturally occurring sRNAs, recent works proposed the use of artificial sRNAs in synthetic biology for predictable repression of the desired genes. Their potential was demonstrated in several application fields, such as metabolic engineering and bacterial physiology studies. Guidelines for the rational design of novel sRNAs have been recently proposed. According to these guidelines, in this work synthetic sRNAs were designed, constructed and quantitatively characterized in Escherichia coli. An sRNA targeting the reporter gene RFP was tested by measuring the specific gene silencing when RFP was expressed at different transcription levels, under the control of different promoters, in different strains, and in single-gene or operon architecture. The sRNA level was tuned by using plasmids maintained at different copy numbers. Results demonstrated that RFP silencing worked as expected in an sRNA and mRNA expression-dependent fashion. A mathematical model was used to support sRNA characterization and to estimate an efficiency-related parameter that can be used to compare the performance of the designed sRNA. Gene silencing was also successful when RFP was placed in a two-gene synthetic operon, while the non-target gene (GFP) in the operon was not considerably affected. Finally, silencing was evaluated for another designed sRNA targeting the endogenous lactate dehydrogenase gene. The quantitative study performed in this work elucidated interesting performance-related and context-dependent features of synthetic sRNAs that will strongly support predictable gene silencing in disparate basic or applied research studies.






Similar content being viewed by others
References
Aiba H (2007) Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol 10(2):134–139
Archer CT, Kim JF, Jeong H, Park JH, Vickers CE, Lee SY, Nielsen LK (2011) The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics 12:9
Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA (2013) Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res 41(15):7429–7437
Canton B, Labno A, Endy D (2008) Refinement and standardization of synthetic biological parts and devices. Nat Biotechnol 26:787–793
Chen S, Zhang A, Blyn LB, Storz G (2004) MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J Bacteriol 186(20):6689–6697
Choudhary E, Thakur P, Pareek M, Agarwal N (2015) Gene silencing by CRISPR interference in mycobacteria. Nat Commun 6:6267
Desnoyers G, Morissette A, Prevost K, Masse E (2009) Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J 28(11):1551–1561
Gay RJ, McComb RB, Bowers GN (1968) Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem 14(8):740–753
Gottesman S (2004) The small RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol 58:303–328
Gottesman S (2005) Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet 21(7):399–404
Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ (2006) A bottom-up approach to gene regulation. Nature 439(7078):856–860
Jost D, Nowojewski A, Levine E (2011) Small RNA biology is systems biology. BMB Rep 44(1):11–21
Keene JD, Tenenbaum SA (2002) Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell 9(6):1161–1167
Kelly JR, Rubin AJ, Davis JH, Ajo-Franklin CM, Cumbers J, Czar MJ, de Mora K, Glieberman AL, Monie DD, Endy D (2009) Measuring the activity of BioBrick promoters using an in vivo reference standard. J Biol Eng 3:4
Knight T (2003) Idempotent vector design for standard assembly of Biobricks. MIT DSpace 2003. http://hdl.handle.net/1721.1/21168
Kosuri S, Goodman DB, Cambray G, Mutalik VK, Gao Y, Arkin AP, Endy D, Church GM (2013) Composability of regulatory sequences controlling transcription and translation in Escherichia coli. PNAS 110(34):14024–14029
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8(11):2180–2196
Lavi-Itzkovitz A, Peterman N, Jost D, Levine E (2014) Quantitative effect of target translation in small RNA efficacy reveals a novel mode of interaction. Nucleic Acids Res 42(19):12200–12211
Lee TS, Krupa RA, Zhang F, Hajimorad M, Holtz WJ, Prasad N, Lee SK, Keasling JD (2011) BglBrick vectors and datasheets: a synthetic biology platform for gene expression. J Biol Eng 5:12
Levine E, Zhang Z, Kuhlman T, Hwa T (2007) Quantitative characteristics of gene regulation by small RNA. PLoS Biol 5(9):e229
Levin-Karp A, Barenholz U, Bareia T, Dayagi M, Zelcbuch L, Antonovsky N, Noor E, Milo R (2013) Quantifying translational coupling in E. coli synthetic operons using RBS modulation and fluorescent reporters. ACS Synth Biol 2(6):327–336
Lutz R, Bujard H (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25(6):1203–1210
Man S, Cheng R, Miao C, Gong Q, Gu Y, Lu X, Han F, Yu W (2011) Artificial trans-encoded small non-coding RNAs specifically silence the selected gene expression in bacteria. Nucleic Acids Res 39(8):e50
Markham MR, Zuker M (2005) DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res 3:W577–W581
Masse E, Gottesman S (2002) A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. PNAS 99(7):4620–4625
Masse E, Escorcia FE, Gottesman S (2003) Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev 17(19):2374–2383
Masse E, Vanderpool CK, Gottesman S (2005) Effect of RyhB small RNA on global iron use in Echerichia coli. J Bacteriol 187(20):6962–6971
Milo R (2013) What is the total number of protein molecules per cell volume? A call to rethink some published values. BioEssays 35(12):1050–1055
Moller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P (2002) Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev 16(13):1696–1706
Morita T, Mochizuki Y, Aiba H (2006) Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. PNAS 103(13):4858–4863
Mutalik VK, Guimaraes JC, Cambray G, Mai QA, Christoffersen MJ, Martin L, Yu A, Lam C, Rodriguez C, Bennett G, Keasling JD, Endy D, Arkin AP (2013) Quantitative estimation of activity and quality for collections of functional genetic elements. Nat Methods 10(4):347–353
Na D, Yoo SM, Chung H, Park H, Park JH, Lee SY (2013) Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat Biotechnol 31(2):170–174
Nakashima N, Tamura T (2009) Conditional gene silencing of multiple genes with antisense RNAs and generation of a mutator strain of Escherichia coli. Nucleic Acids Res 37(15):e103
Nakashima N, Tamura T, Good L (2006) Paired termini stabilize antisense RNAs and enhance conditional gene silencing in Escherichia coli. Nucleic Acids Res 34(20):e138
Pasotti L, Zucca S (2014) Advances and computational tools towards predictable design in biological engineering. Comput Math Methods Med 2014:369681. doi:10.1155/2014/369681
Pasotti L, Politi N, Zucca S, Cusella De Angelis MG, Magni P (2012) Bottom-up engineering of biological systems through standard bricks: a modularity study on basic parts and devices. PLoS ONE 7(7):e39407
Pasotti L, Zucca S, Magni P (2013) Modelling for synthetic biology. In: Modeling methodology for physiology and medicine: second edition. Elsevier, pp 545–564. doi:10.1016/B978-0-12-411557-6.00023-9
Peterman N, Lavi-Itzkovitz A, Levine E (2014) Large-scale mapping of sequence-function relations in small regulatory RNAs reveals plasticity and modularity. Nucleic Acids Res 42(19):12177–12188
Pfeiffer V, Papenfort K, Lucchini S, Hinton JC, Vogel J (2009) Coding sequence targeting by MicC RNA reveals bacterial mRNA silencing downstream of translational initiation. Nat Struct Mol Biol 16(8):840–846
Politi N, Pasotti L, Zucca S, Casanova M, Micoli G, Cusella De Angelis MG, Magni P (2014) Half-life measurements of chemical inducers for recombinant gene expression. J Biol Eng 8:5
Politi N, Pasotti L, Zucca S, Magni P (2015) Modelling the effects of cell-to-cell variability on the output of interconnected gene networks in bacterial populations. BMC Syst Biol 9(Suppl 3):S6. doi:10.1186/1752-0509-9-S3-S6
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152(5):1173–1183
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Selinger DW, Saxena RM, Cheung KJ, Church GM, Rosenow C (2003) Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation. Genome Res 13(2):216–223
Sharma V, Yamamura A, Yokobayashi Y (2012) Engineering artificial small RNAs for conditional gene silencing in Escherichia coli. ACS Synth Biol 1(1):6–13
Sharma V, Sakai Y, Smythe KA, Yokobayashi Y (2013) Knockdown of recA gene expression by artificial small RNAs in Escherichia coli. Biochem Biophys Res Commun 430(1):256–259
Shetty R, Endy D, Knight T (2008) Engineering BioBrick vectors from BioBrick parts. J Biol Eng 2:5
Shimoni Y, Friedlander G, Hetzroni G, Niv G, Altuvia S, Biham O, Margalit H (2007) Regulation of gene expression by small non-coding RNAs: a quantitative view. Mol Syst Biol 3:138
Tummala SB, Welker NE, Papoutsakis ET (2003) Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J Bacteriol 185(6):1923–1934
Urban JH, Vogel J (2007) Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res 35(3):1018–1037
Wang B, Kitney RI, Joly N, Buck M (2011) Engineering modular and orthogonal genetic logic gates for robust digital-like synthetic biology. Nat Commun 2:508
Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S (2001) Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev 15(13):1637–1651
Yoo SM, Na D, Lee SY (2013) Design and use of synthetic regulatory small RNAs to control gene expression in Escherichia coli. Nat Protoc 8(9):1694–1707
Zucca S, Pasotti L, Mazzini G, De Angelis MG, Magni P (2012) Characterization of an inducible promoter in different DNA copy number conditions. BMC Bioinform 13(Suppl 4):S11
Zucca S, Pasotti L, Politi N, Cusella De Angelis MG, Magni P (2013) A standard vector for the chromosomal integration and characterization of BioBrick™ parts in Escherichia coli. J Biol Eng 7:12
Zucca S, Pasotti L, Politi N, Casanova M, Mazzini G, Cusella De Angelis MG, Magni P (2015) Multi-faceted characterization of a novel LuxR-repressible promoter library for Escherichia coli. PLoS ONE 10(5):e0126264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
Raw data and processed time series for cultures in representative experiments. A Raw absorbance time series of sterile medium (M9) and a non-fluorescent culture (TOP10). B Background-subtracted absorbance (OD 600) time series of a non- luorescent culture (TOP10). C Raw red fluorescence of sterile medium (M9) and a nonfluorescent culture (TOP10) time series, showing that the autofluorescence of bacteria is comparable with the fluorescence of medium and it is not OD 600-dependent. D Raw green fluorescence of sterile medium (M9) and a nonfluorescent culture (TOP10) time series, showing that the autofluorescence of bacteria is higher than the fluorescence of medium and it is OD 600-dependent. E Raw green fluorescence as a function of OD 600 for several non-fluorescent strains assayed in the same experiment; such data are used to compute the OD 600-dependent autofluorescence function (by linear regression), which represents the background green fluorescence at a given OD 600; circles represent data points and solid line represents the regression line. F Raw absorbance time series of three RFP-expressing cultures: Plux-R, sRFP-1A2+Plux-R and J101-R in TOP10. G Background-subtracted OD 600 time series of the three RFPexpressing cultures. H Raw red fluorescence time series of the three RFP-expressing cultures. I Background subtracted red fluorescence time series of the three RFP-expressing cultures, yielding a time series proportional to the total RFP proteins in the microplate well. J Numeric time derivative of RFP divided by OD 600, yielding a signal proportional to the RFP synthesis rate per cell at the steady-state; the time series in the exponential growth phase (OD 600 between 0.05 and 0.18, assumed) is shown for the three RFP-expressing cultures; for each culture, this time series is averaged and divided by the average RFP synthesis rate per cell of the reference culture (see Methods section in the main text). K–O the same time series as panels F–J are shown for two GFP-expressing cultures: Plux-G and sRFP-1A2+Plux-G (PNG 1485 kb)
Supplementary material 2
Doubling times of recombinant strains bearing a single-gene RFP or GFP expression system driven by Plux (Plux-R or Plux-G). A Specific silencing of the target gene (RFP) via sRFP in TOP10 and W. B Unspecific silencing of RFP or GFP via different sRNAs in TOP10 and W. Bars represent the mean doubling time value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value (PNG 1350 kb)
Supplementary material 3
Doubling times of recombinant strains bearing an RFP expression system driven by BBa_J23101 (J101-R). Bars represent the mean doubling time value computed on at least three biological replicates in the indicated conditions. Error bars represent the 95 % confidence intervals of the mean value (PNG 285 kb)
Supplementary material 4
Silencing results for RFP expressed by a single-gene cassette (J101-R32) driven by BBa_J23101 with the BBa_B0032 RBS upstream of the RFP gene. A Specific silencing of the target gene (RFP) via sRFP in TOP10. Bars represent the mean Scell value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value. Asterisks indicate that the Scell value in the condition is statistically different from the Scell of the expression cassette without sRNAs (J101-R32). Percentages represent the Eff% values. B Doubling times (PNG 509 kb)
Supplementary material 5
Doubling times of recombinant TOP10 bearing the Plux-RG and Plux-GR synthetic operons. A Silencing of the target gene (RFP) and the non-target gene (GFP) via the silencing device sRFP. B Unspecific silencing of RFP and GFP, in the Plux-GR construct, via different sRNAs. Bars represent the mean doubling time value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value (PNG 604 kb)
Supplementary material 6
Silencing results for RFP and GFP expressed by the Plux-GR synthetic operon in the W strain. A Silencing of the target gene (RFP) and the non-target gene (GFP) via the silencing device sRFP. B Unspecific silencing of RFP and GFP via different sRNAs. Bars represent the mean Scell value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value. Asterisks indicate that the Scell value of RFP or GFP in the condition is statistically different from the Scell of the operon without sRNAs (Plux-GR). Percentages represent the Eff% values. When Scell in a given condition is higher than Scell without sRNA, Eff% value is set to zero (PNG 695 kb)
Supplementary material 7
Doubling times of recombinant W bearing the Plux-GR synthetic operon. A Specific silencing of the target gene (RFP) and the non-target gene (GFP) via the silencing device sRFP. B Unspecific silencing of RFP and GFP via different sRNAs. Bars represent the mean doubling time value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value (PNG 576 kb)
Supplementary material 8
Doubling times of recombinant strains bearing the Plux-RG30 and Plux-G30R synthetic operons in TOP10 and W. A Silencing of the target gene (RFP) and the non-target gene (GFP) via the silencing device sRFP. B Unspecific silencing of RFP and GFP via different sRNAs. Bars represent the mean doubling time value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value (PNG 1387 kb)
Supplementary material 9
Silencing results for RFP and GFP expressed by the Plux-RG30 and Plux-G30R synthetic operons in TOP10 and W. A Silencing of the target gene (RFP) and the non-target gene (GFP) via the silencing device sRFP. B Unspecific silencing of RFP and GFP via different sRNAs. Bars represent the mean Scell value computed on at least three biological replicates. Error bars represent the 95 % confidence intervals of the mean value. Asterisks indicate that the Scell value of RFP or GFP in the condition is statistically different from the Scell of the operon without sRNA (Plux-RG30 or Plux-G30R). Percentages represent the Eff% values. When Scell in a given condition is higher than Scell without sRNA, Eff% value is set to zero (PNG 2686 kb)
Rights and permissions
About this article
Cite this article
Massaiu, I., Pasotti, L., Casanova, M. et al. Quantification of the gene silencing performances of rationally-designed synthetic small RNAs. Syst Synth Biol 9, 107–123 (2015). https://doi.org/10.1007/s11693-015-9177-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11693-015-9177-7