Abstract
Purpose
Avian haemosporidian may affect the host from body damage to the extinction of a population. Knowledge of their status may help in future avifauna conservation plans. Hence, their status in two bird groups of India and their phylogenetic relationships with other known lineages of the world were examined.
Methods
Cytochrome b gene sequences (479 bp) generated from India and available at MalAvi database were used to study the avian haemosporidian prevalence and phylogenetic analysis of lineages at local and world levels.
Results
One common (COLL2) and only once in the study (CYOPOL01, CHD01, CYORUB01, EUMTHA01, GEOCIT01) haemosporidian lineages were discovered. 5.88% prevalence of haemosporidian infection was found in 102 samples belonging to 6 host species. Haemoproteus prevalence was 4.90% across five host species (Phylloscopus trochiloides, Cyornis poliogenys, C. hainanus dialilaemus, C. rubeculoides, Eumiyas thalassinus) and Plasmodium prevalence was 0.98% in Geokichla citrina. Spatial phylogeny at the global level showed that COLL2 lineage, found in C. poliogenys in India, was genetically identical to H. pallidus lineages (COLL2) in parts of Africa, Europe, North America, Malaysia, and the Philippines. The Plasmodium lineage (GEOCIT01) was related to PADOM16 in Egypt, but the sequences were only 93.89% alike.
Conclusions
Four new lineages of Haemoproteus and one of Plasmodium were reported. COLL2 similarity with other H. pallidus lineages may suggest their hosts as possible infection sources.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Atkinson CT, Van RC (1991) Pathogenicity and epizootiology of avian haematozoa. Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird-parasite interactions ecology, evolution, and behaviour. Oxford University Press, Oxford, pp 19–48
Valkiunas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton Florida, p 932
Ilgunas M, Bukauskaite D, Palinauskas V, Iezhova T, Fragner K, Platonova E, Weissenböck H, Valki unas G (2019) Patterns of Plasmodium homocircumflexum virulence in experimentally infected passerine birds. Malar J 18:174
Muriel J, Marzal A, Magallanes S, García-Longoria L, Suarez-Rubio M, Bates PJJ, Lin HH, Soe AN, Oo KS, Aye AA et al (2021) Prevalence and diversity of avian haemosporidians may vary with anthropogenic disturbance. Diversity 13:111
Valkiunas G, Atkinson CT (2020) Introduction to life cycles, taxonomy, distribution, and basic research techniques. In: Santiago-Alarcon D, Marzal A (eds) avian malaria and related parasites in the tropics. Springer International Publishing, Cham, Switzerland, pp 45–80
Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347:436–438
Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665
Marzal A, Balbontín J, Reviriego M, García-Longoria L, Relinque C, Hermosell IG, Magallanes S, López-Calderón C, De Lope F, Møller AP (2016) A longitudinal study of age-related changes in Haemoproteus infection in a passerine bird. Oikos 125:1092–1099
Marzal A, De Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc R Soc B Biol Sci 267:2507–2510
Marzal A, Bensch S, Reviriego M, Balbontin J, De Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21:979–987
Palinauskas V, Valkiunas G, Bolshakov CV, Bensch S (2008) Plasmodium relictum (lineage P-SGS1): Effects on experimentally infected passerine birds. Exp Parasitol 120:372–380
Lapointe DA, Atkinson CT, Samuel MD (2012) Ecology and conservation biology of avian malaria. Ann N Y Acad Sci 1249:211–226
Marzal A, Garcia-Longoria L (2020) The role of malaria parasites in invasion biology. Avian malaria and related parasites in the tropics. Springer International Publishing Cham, Switzerland, pp 487–512
Rahmana A R Bird diversity of India. http://bnhsenvis.nic.in/files/Bird_Diversity_Popular_Lecture.pdf
Farah I (2017) Exploring host and geographical shifts in transmission of haemosporidians in a Palaearctic passerine wintering in India. J Ornithol 158:869–874. https://doi.org/10.1007/s10336-017-1444-9
Gupta P, Vishnudas CK, Ramakrishnan U, Robin VV, Dharmarajan G (2019) Geographical and host species barriers differentially affect generalist and specialist parasite community structure in a tropical skyisland archipelago. Proc R Soc B 286:20190439. https://doi.org/10.1098/rspb.0439
Bensch S, Hellgren O, Perez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. https://doi.org/10.1111/j.1755-0998.2009.02692.x
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. Proceedings of the Royal Society of London. Series B Biological Sciences 269: 885–892
Beadell JS, Gering E, Austin J, Dumbacher JP, Peirce M, Thane AK, Carter PAT, Robert FC (2004) Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol Ecol 13:3829–3844
Marzal A, Ricklefs RE, Valkiunas G, Albayrak T, Arriero E, Bonneaud C, Czirják GA, Ewen J, Hellgren O, Hořáková D, Iezhova TA, Jensen H, Križanauskienė A, Lima MR, de Lope F, Magnussen E, Martin LB, Møller AP, Palinauskas V, Pap PL, Pérez-Tris J, Sehgal RNM, Soler M, Szöllősi E, Westerdahl H, Zetindjiev P, Bensch S (2011) Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 6(7):e21905. https://doi.org/10.1371/journal.pone.0021905
Jia T, Huang X, Valkiūnas G, Yang M, Zheng C, Tianchun Pu, Yanyun Zhang Lu, Dong XS, Zhang C (2018) Malaria parasites and related haemosporidians cause mortality in cranes: a study on the parasites diversity, prevalence and distribution in Beijing Zoo. Malar J 17:234. https://doi.org/10.1186/s12936-018-2385-3
Gil-Vargas DL, Sedano-Cruz RE (2019) Genetic variation of avian malaria in the tropical Andes: a relationship with the spatial distribution of hosts. Malar J 18:129. https://doi.org/10.1186/s12936-019-2699-9
Bensch S, Stjernman M, Ostman HDO, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc London B Biol Sci 267:1583–1589
Winkler DW, Billerman SM, Lovette IJ (2020) Old World Flycatchers (Muscicapidae), version 10. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the World. Cornell Lab of Ornithology, Ithaca
Levin II, Colborn RE, Kim D, Perlut NG, Renfrew RB, Parker PG (2016) Local parasite lineage sharing in temperate grassland birds provides clues about potential origins of Galapagos avian Plasmodium. Ecol Evol 6:716–726
Singh A, Gupta SK, Alström P, Mohan D, Hooper DM, Kumar RS, Bhatt D, Singh P, Price TD (2019) Taxonomy of cryptic species in the Cyornis rubeculoides complex in the Indian subcontinent. Ibis 162(3):924–935. https://doi.org/10.1111/ibi.12735
Singh A, Kumar A, Kumar RS, Bhatt D, Gupta SK (2017) Amplification of mtDNA control region in opportunistically collected bird samples belonging to nine families of the order Passeriformes. Mitochondrial DNA Part B 2(1):99–100. https://doi.org/10.1080/23802359.2017.1289342
Hellgren O, Krizanauskiene A, Valkiunas G, Bensch S (2017) Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus (Haemosporida, Haemoproteidae). J Parasitol 93:889–896
Tan T, M C, Nelson J S, H C Ng, R C Y Ting, and U A K Kara, (1997) Direct PCR amplification and sequence analysis of extra chromosomal Plasmodium DNA from dried blood spots. Acta Trop 68:105–114
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7(1–2):203–214
Hellgren O (2005) The occurrence of haemosporidian parasites in the Fennoscandian bluethroat (Luscinia svecica) population. J Ornithol 146:55–60
Chagas CRF, Valkiūnas G, de Oliveira GL, Monteiro EF, Guida FJV, Simões RF, Rodrigues PT, de Albuquerque Luna EJ, Kirchgatter K (2017) Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar J 16:83
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Fábio K, Mendes Nicola F, Müller Huw A, Ogilvie L, du Plessis A, Popinga AR, Rasmussen D, Siveroni I, Suchard MA, Chieh-Hsi W, Xie D, Zhang C, Stadler T, Drummond AJ (2019) BEAST 25 An advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15(4):e1006650
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 17. Syst Biol. https://doi.org/10.1093/sysbio/syy032
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Rambaut A (2018) FigTree v1.4.4, A Graphical Viewer of Phylogenetic Trees. Available from 〈http://tree.bio.ed.ac.uk/software/figtree/〉.
Bouckaert R (2016) Phylogeography by diffusion on a sphere: whole world phylogeography. PeerJ 4:e2406. https://doi.org/10.7717/peerj.2406
Filip B, Guy B, Bram V, Suchard Marc A, Andrew R, Philippe L (2016) SpreaD3: interactive visualisation of spatiotemporal history and trait evolutionary processes. Mol Biol Evol 33(8):2167–2169. https://doi.org/10.1093/molbev/msw082
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797
BirdLife International (2017) Cyornis rubeculoides (amended version of 2016 assessment) The IUCN Red List of Threatened Species 2017: e.T103761873A111163294. https://doi.org/10.2305/IUCN.UK.2017-1.RLTS.T103761873A111163294.en. Downloaded on 25 July 2020
BirdLife International. Cyornis poliogenys (2018) The IUCN Red List of Threatened Species. e.T22709527A131953768. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22709527A131953768.en. Downloaded on 25 July 2020
Aparup D, Anvikara AR, Catorb LJ, Dhimana RC, Eapenc A, Mishraa N, Nagpala BN, Nandaa N, Raghavendraa K, Readd AF, Sharmae SK, Singha OP, Singha V, Sinnisf P, Srivastavag HC, Sullivanh SA, Suttonh PL, Thomasb MB, Carltonh JM, Valecha N (2012) Malaria in India: the center for the study of complex malaria in India. Acta Trop 121(3):267–273. https://doi.org/10.1016/j.actatropica.2011.11.008
Foster WA, Walker ED (2002) MOSQUITOES (Culicidae) (Eds). Gary mullen, lance durden medical and veterinary entomology. Academic Press, Cambridge, pp 203–262
Pigeault R, Vézilier J, Cornet S, Zélé F, Nicot A, Perret P, Gandon S (2015) Rivero A (2014) avian malaria: a new lease of life for an old experimental model to study the evolutionary ecology of Plasmodium. Phil Trans R Soc B 370:20140300. https://doi.org/10.1098/rstb.2014.0300
Adie MRS and Adie JR (1912) Note of an inquiry into malaria and mosquitoes in the Kashmir valley. The Indian Medical Gazette 1913 Page 341
Dar TH (2018) Faunistic studies on the diversity and distribution of mosquitoes of the high altitude Himalayan Region Jammu and Kashmir. A Ph. D thesis submitted to the Pondicherry University. URI: http://hdl.handle.net/10603/284347
Puteri R, Abd AM, Coleman GT, Irwin PJ, Traub RJ (2011) Hippobosca longipennis-a potential intermediate host of a species of Acanthocheilonema in dogs in northern India. Parasit Vectors 4:143
Sikha G, Uttaran M, Abhijit M, Chaudhuri Prasanta K (2008) Biting flies of the genus Homohelea of India (Diptera: Ceratopogonidae) 2009. Folia Heyrovskyana series A 16(4):91–106 (ed March 31 2009 ISSN 1801-7142)
Acknowledgements
The current study was part of the project under UGC-Dr. D.S. Kothari Postdoctoral Fellowship Scheme, UGC awarded to the first author (BL/16-17/0151) from March 2017 to March 2020. We acknowledge the Director and Dean, WII for their support. We thank the National Institute of Malaria Research, Dwarka, New Delhi, for sparing the positive Plasmodium DNA sample for the standardization work and the Director, Indian Institute of Integrative Medicine, CSIR, Jammu, for allowing us to carry out the standardization work of this project. We sincerely thank Professor Trevor Price, the University of Chicago, for his generous support. We thank the Forest department of Union Territory of Jammu and Kashmir, Himachal Pradesh, Uttarakhand, Sikkim, Arunachal Pradesh, Mizoram, Andhra Pradesh, and Meghalaya for granting permission for sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11686_2022_626_MOESM1_ESM.jpg
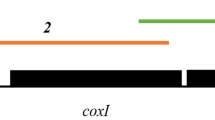
Supplementary file1 Cytochrome b gene (479 bp) [24] sequence comparison for the lineages from MalAvi database (COLL2 and PFC1) and from the current study (COLL2 (LM10), CYOPOL01 (LM6), GEOCIT01 (LM16), CHD01 (AS 86), CYORUB01 (AS91) and EUMTHA01 ( AS102)). Dots represent the common nucleotides. (JPG 529 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vipin, Singh, A., Sharma, V. et al. Avian Haemosporidian (Plasmodium and Haemoproteus) Status in Two Bird Groups (Old-World Flycatchers and Thrushes) of India and Their Phylogenetic Relationships with Other Lineages of the World. Acta Parasit. 67, 1756–1766 (2022). https://doi.org/10.1007/s11686-022-00626-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00626-1