Abstract
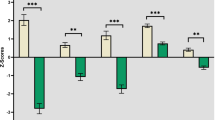
Cognitive impairment is now recognized in a subset of patients with amyotrophic lateral sclerosis (ALS). The objective of the study was to identify group differences and neuroanatomical correlates of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) in participants ALS. Fifty-three ALS patients and 43 healthy controls recruited as a part of our multicentre study (CALSNIC) were administered the ECAS and underwent an MRI scan. Voxel-based morphometry and tract based spatial statistics (TBSS) was performed to identify structural changes and associations with impaired ECAS scores. Lower performance in the ECAS verbal fluency and executive domains were noted in ALS patients as compared to controls (p < 0.01). Extensive white matter degeneration was noted in the corticospinal tract in all ALS patients, while ALS patients with impaired verbal fluency or executive domains (ALS-exi, n = 22), displayed additional degeneration in the corpus callosum, cingulum and superior longitudinal fasciculus as compared to controls (p < 0.05, TFCE corrected). Mild grey matter changes and associations with ECAS verbal fluency or executive performance were noted at lenient statistical thresholds (p < 0.001, uncorrected). Executive impairment was detected using the ECAS in our multicentre sample of Canadian ALS patients. White matter degeneration in motor regions was revealed in ALS patients with extensive spread to frontal regions in the ALS-exi sub-group. Mild associations between ECAS verbal fluency, executive function scores and MRI metrics suggest that reduced performance may be associated with widespread structural integrity.




Similar content being viewed by others
References
Abdulla, S., Machts, J., Kaufmann, J., Patrick, K., Kollewe, K., Dengler, R., Heinze, H. J., Petri, S., Vielhaber, S., & Nestor, P. J. (2014). Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiology of Aging, 35(11), 2639–2645. https://doi.org/10.1016/j.neurobiolaging.2014.05.035.
Abe, K., Fujimura, H., Toyooka, K., Sakoda, S., Yorifuji, S., & Yanagihara, T. (1997). Cognitive function in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 148(1), 95–100.
Abrahams, S., Goldstein, L. H., Al-Chalabi, A., Pickering, A., Morris, R. G., Passingham, R. E., et al. (1997). Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. Journal of Neurol Neurosurg Psychiatry, 62(5), 464–472.
Abrahams, S., Leigh, P. N., Harvey, A., Vythelingum, G. N., Grise, D., & Goldstein, L. H. (2000). Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia, 38(6), 734–747.
Abrahams, S., Newton, J., Niven, E., Foley, J., & Bak, T. H. (2014). Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener, 15(1–2), 9–14. https://doi.org/10.3109/21678421.2013.805784.
Agosta, F., Rocca, M. A., Valsasina, P., Sala, S., Caputo, D., Perini, M., Salvi, F., Prelle, A., & Filippi, M. (2009). A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. Journal of Neurology, Neurosurgery, and Psychiatry, 80(1), 53–55. https://doi.org/10.1136/jnnp.2008.154252.
Agosta, F., Pagani, E., Petrolini, M., Caputo, D., Perini, M., Prelle, A., Salvi, F., & Filippi, M. (2010). Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. AJNR. American Journal of Neuroradiology, 31(8), 1457–1461. https://doi.org/10.3174/ajnr.A2105.
Agosta, F., Ferraro, P. M., Riva, N., Spinelli, E. G., Chio, A., Canu, E., et al. (2016). Structural brain correlates of cognitive and behavioral impairment in MND. Human Brain Mapping, 37(4), 1614–1626. https://doi.org/10.1002/hbm.23124.
Bach, M., Laun, F. B., Leemans, A., Tax, C. M., Biessels, G. J., Stieltjes, B., et al. (2014). Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage, 100, 358–369. https://doi.org/10.1016/j.neuroimage.2014.06.021.
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15(7–8), 435–455. https://doi.org/10.1002/nbm.782.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck depression inventory-II. San Antonio: Psychological Corporation.
Bede, P., Bokde, A., Elamin, M., Byrne, S., McLaughlin, R. L., Jordan, N., Hampel, H., Gallagher, L., Lynch, C., Fagan, A. J., Pender, N., & Hardiman, O. (2013). Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. Journal of Neurology, Neurosurgery, and Psychiatry, 84(7), 766–773. https://doi.org/10.1136/jnnp-2012-302674.
Birn, R. M., Kenworthy, L., Case, L., Caravella, R., Jones, T. B., Bandettini, P. A., & Martin, A. (2010). Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage, 49(1), 1099–1107. https://doi.org/10.1016/j.neuroimage.2009.07.036.
Braak, H., Brettschneider, J., Ludolph, A. C., Lee, V. M., Trojanowski, J. Q., & Del Tredici, K. (2013). Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nature Reviews. Neurology, 9(12), 708–714. https://doi.org/10.1038/nrneurol.2013.221.
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossman, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology, 74(1), 20–38. https://doi.org/10.1002/ana.23937.
Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1(5), 293–299.
Burke, T., Lonergan, K., Pinto-Grau, M., Elamin, M., Bede, P., Madden, C., Hardiman, O., & Pender, N. (2017). Visual encoding, consolidation, and retrieval in amyotrophic lateral sclerosis: Executive function as a mediator, and predictor of performance. Amyotroph Lateral Scler Frontotemporal Degener, 18(3–4), 193–201. https://doi.org/10.1080/21678421.2016.1272615.
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., & Nakanishi, A. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). Journal of the Neurological Sciences, 169(1–2), 13–21.
Chang, J. L., Lomen-Hoerth, C., Murphy, J., Henry, R. G., Kramer, J. H., Miller, B. L., & Gorno-Tempini, M. L. (2005). A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology, 65(1), 75–80. https://doi.org/10.1212/01.wnl.0000167602.38643.29.
Christidi, F., Karavasilis, E., Riederer, F., Zalonis, I., Ferentinos, P., Velonakis, G., Xirou, S., Rentzos, M., Argiropoulos, G., Zouvelou, V., Zambelis, T., Athanasakos, A., Toulas, P., Vadikolias, K., Efstathopoulos, E., Kollias, S., Karandreas, N., Kelekis, N., & Evdokimidis, I. (2018). Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: A combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging and Behavior, 12(2), 547–563. https://doi.org/10.1007/s11682-017-9722-y.
Dimond, D., Ishaque, A., Chenji, S., Mah, D., Chen, Z., Seres, P., Beaulieu, C., & Kalra, S. (2017). White matter structural network abnormalities underlie executive dysfunction in amyotrophic lateral sclerosis. Human Brain Mapping, 38(3), 1249–1268. https://doi.org/10.1002/hbm.23452.
Goldstein, L. H., & Abrahams, S. (2013). Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurology, 12(4), 368–380. https://doi.org/10.1016/S1474-4422(13)70026-7.
Kasper, E., Schuster, C., Machts, J., Kaufmann, J., Bittner, D., Vielhaber, S., Benecke, R., Teipel, S., & Prudlo, J. (2014). Microstructural white matter changes underlying cognitive and behavioural impairment in ALS--an in vivo study using DTI. PLoS One, 9(12), e114543. https://doi.org/10.1371/journal.pone.0114543.
Kaufmann, P., Pullman, S. L., Shungu, D. C., Chan, S., Hays, A. P., Del Bene, M. L., et al. (2004). Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS). Neurology, 62(10), 1753–1757.
Keller, J., Bohm, S., Aho-Ozhan, H. E. A., Loose, M., Gorges, M., Kassubek, J., et al. (2017). Functional reorganization during cognitive function tasks in patients with amyotrophic lateral sclerosis. Brain Imaging and Behavior, 12, 771–784. https://doi.org/10.1007/s11682-017-9738-3.
Kew, J. J., Goldstein, L. H., Leigh, P. N., Abrahams, S., Cosgrave, N., Passingham, R. E., et al. (1993). The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain, 116(Pt 6), 1399–1423.
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., Burrell, J. R., & Zoing, M. C. (2011). Amyotrophic lateral sclerosis. Lancet, 377(9769), 942–955. https://doi.org/10.1016/S0140-6736(10)61156-7.
Kimura, F., Fujimura, C., Ishida, S., Nakajima, H., Furutama, D., Uehara, H., Shinoda, K., Sugino, M., & Hanafusa, T. (2006). Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology, 66(2), 265–267. https://doi.org/10.1212/01.wnl.0000194316.91908.8a.
Leemans, A, Jeurissen, B, Sijbers, J, and Jones, DK (2009). ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Paper presented at the 17th annual meeting of Intl Soc mag Reson med, Hawaii, USA,
Loose, M, Burkhardt, C, Aho-Ozhan, H, Keller, J, Abdulla, S, Bohm, S, et al. (2016). Age and education-matched cut-off scores for the revised German/Swiss-German version of ECAS. Amyotroph Lateral Scler Frontotemporal Degener, 1-3, doi:https://doi.org/10.3109/21678421.2016.1162814.
Lulé, D., Burkhardt, C., Abdulla, S., Bohm, S., Kollewe, K., Uttner, I., et al. (2015). The Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen: A cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener, 16(1–2), 16–23. https://doi.org/10.3109/21678421.2014.959451.
Lulé, D., Bohm, S., Muller, H. P., Aho-Ozhan, H., Keller, J., Gorges, M., et al. (2018). Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex, 101, 163–171. https://doi.org/10.1016/j.cortex.2018.01.004.
Machts, J., Bittner, V., Kasper, E., Schuster, C., Prudlo, J., Abdulla, S., Kollewe, K., Petri, S., Dengler, R., Heinze, H. J., Vielhaber, S., Schoenfeld, M. A., & Bittner, D. M. (2014). Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: A comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neuroscience, 15, 83.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239.
Menke, R. A., Korner, S., Filippini, N., Douaud, G., Knight, S., Talbot, K., et al. (2014). Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain, 137(Pt 9), 2546–2555.
Menke, R. A., Proudfoot, M., Wuu, J., Andersen, P. M., Talbot, K., Benatar, M., et al. (2016). Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk. Journal of Neurology, Neurosurgery, and Psychiatry, 87(6), 580–588. https://doi.org/10.1136/jnnp-2015-311945.
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R., & Hodges, J. R. (2006). The Addenbrooke's cognitive examination revised (ACE-R): A brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry, 21(11), 1078–1085. https://doi.org/10.1002/gps.1610.
Mioshi, E., Lillo, P., Yew, B., Hsieh, S., Savage, S., Hodges, J. R., Kiernan, M. C., & Hornberger, M. (2013). Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology, 80(12), 1117–1123.
Mitsumoto, H., Ulug, A. M., Pullman, S. L., Gooch, C. L., Chan, S., Tang, M. X., et al. (2007). Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology, 68(17), 1402–1410. https://doi.org/10.1212/01.wnl.0000260065.57832.87.
Müller, H. P., Turner, M. R., Grosskreutz, J., Abrahams, S., Bede, P., Govind, V., Prudlo, J., Ludolph, A. C., Filippi, M., & Kassubek, J. (2016). A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 87(6), 570–579. https://doi.org/10.1136/jnnp-2015-311952.
Niven, E., Newton, J., Foley, J., Colville, S., Swingler, R., Chandran, S., Bak, T. H., & Abrahams, S. (2015). Validation of the Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen (ECAS): A cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener, 16(3–4), 172–179. https://doi.org/10.3109/21678421.2015.1030430.
Phukan, J., Elamin, M., Bede, P., Jordan, N., Gallagher, L., Byrne, S., Lynch, C., Pender, N., & Hardiman, O. (2012). The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. Journal of Neurology & Psychiatry, 83(1), 102–108.
Pinto-Grau, M., Burke, T., Lonergan, K., McHugh, C., Mays, I., Madden, C., Vajda, A., Heverin, M., Elamin, M., Hardiman, O., & Pender, N. (2017). Screening for cognitive dysfunction in ALS: Validation of the Edinburgh cognitive and Behavioural ALS screen (ECAS) using age and education adjusted normative data. Amyotroph Lateral Scler Frontotemporal Degener, 18(1–2), 99–106. https://doi.org/10.1080/21678421.2016.1249887.
Poletti, B., Solca, F., Carelli, L., Madotto, F., Lafronza, A., Faini, A., Monti, A., Zago, S., Calini, D., Tiloca, C., Doretti, A., Verde, F., Ratti, A., Ticozzi, N., Abrahams, S., & Silani, V. (2016). The validation of the Italian Edinburgh cognitive and Behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener, 17(7–8), 489–498. https://doi.org/10.1080/21678421.2016.1183679.
Sarro, L., Agosta, F., Canu, E., Riva, N., Prelle, A., Copetti, M., Riccitelli, G., Comi, G., & Filippi, M. (2011). Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: A diffusion tensor tractography study. Ajnr: American Journal of Neuroradiology, 32(10), 1866–1872.
Schuster, C., Kasper, E., Dyrba, M., Machts, J., Bittner, D., Kaufmann, J., Mitchell, A. J., Benecke, R., Teipel, S., Vielhaber, S., & Prudlo, J. (2014). Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiology of Aging, 35(1), 240–246. https://doi.org/10.1016/j.neurobiolaging.2013.07.020.
Senda, J., Ito, M., Watanabe, H., Atsuta, N., Kawai, Y., Katsuno, M., Tanaka, F., Naganawa, S., Fukatsu, H., & Sobue, G. (2009). Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: A study with tractography and diffusion-tensor imaging. Amyotrophic Lateral Sclerosis, 10(5–6), 288–294. https://doi.org/10.3109/17482960802651717.
Sgobio, C., Trabalza, A., Spalloni, A., Zona, C., Carunchio, I., Longone, P., & Ammassari-Teule, M. (2008). Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes, Brain, & Behavior, 7(4), 427–434.
Siciliano, M., Trojano, L., Trojsi, F., Greco, R., Santoro, M., Basile, G., Piscopo, F., D’Iorio, A., Patrone, M., Femiano, C., Monsurrò, M., Tedeschi, G., & Santangelo, G. (2017). Edinburgh cognitive and Behavioural ALS screen (ECAS)-Italian version: Regression based norms and equivalent scores. Neurological Sciences, 38(6), 1059–1068. https://doi.org/10.1007/s10072-017-2919-4.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–1722.
Strong, M. J., Abrahams, S., Goldstein, L. H., Woolley, S., McLaughlin, P., Snowden, J., et al. (2017). Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener, 18(3–4), 153–174. https://doi.org/10.1080/21678421.2016.1267768.
Taylor, L. J., Brown, R. G., Tsermentseli, S., AlChalabi, A., Shaw, C. E., Ellis, C. M., et al. (2013). Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? Journal of Neurology & Psychiatry, 84(5), 494–498.
Trojsi, F., Caiazzo, G., Siciliano, M., Femiano, C., Passaniti, C., Russo, A., Bisecco, A., Monsurrò, M. R., Cirillo, M., Esposito, F., Tedeschi, G., & Santangelo, G. (2019). Microstructural correlates of Edinburgh cognitive and Behavioural ALS screen (ECAS) changes in amyotrophic lateral sclerosis. Psychiatry Research: Neuroimaging, 288, 67–75. https://doi.org/10.1016/j.pscychresns.2019.04.001.
Turner, M. R., Grosskreutz, J., Kassubek, J., Abrahams, S., Agosta, F., Benatar, M., Filippi, M., Goldstein, L. H., van den Heuvel, M., Kalra, S., Lulé, D., Mohammadi, B., & first Neuroimaging Symosium in ALS (NISALS). (2011). Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurology, 10(5), 400–403. https://doi.org/10.1016/S1474-4422(11)70049-7.
Witgert, M., Salamone, A. R., Strutt, A. M., Jawaid, A., Massman, P. J., Bradshaw, M., Mosnik, D., Appel, S. H., & Schulz, P. E. (2010). Frontal-lobe mediated behavioral dysfunction in amyotrophic lateral sclerosis. European Journal of Neurology, 17(1), 103–110.
Ye, S., Ji, Y., Li, C., He, J., Liu, X., & Fan, D. (2016). The Edinburgh cognitive and Behavioural ALS screen in a Chinese amyotrophic lateral sclerosis population. PLoS One, 11(5), e0155496. https://doi.org/10.1371/journal.pone.0155496.
Zhang, H., Avants, B. B., Yushkevich, P. A., Woo, J. H., Wang, S., McCluskey, L. F., et al. (2007a). High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Transactions on Medical Imaging, 26(11), 1585–1597. https://doi.org/10.1109/TMI.2007.906784.
Zhang, H., Yushkevich, P. A., Rueckert, D., & Gee, J. C. (2007b). Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv, 10(Pt 2), 211–218.
Zhang, Y., Schuff, N., Woolley, S. C., Chiang, G. C., Boreta, L., Laxamana, J., Katz, J. S., & Weiner, M. W. (2011). Progression of white matter degeneration in amyotrophic lateral sclerosis: A diffusion tensor imaging study. Amyotrophic Lateral Sclerosis, 12(6), 421–429. https://doi.org/10.3109/17482968.2011.593036.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
SK received funding from the Canadian Institutes of Health Research (CIHR) Operating grant (CIHR MOP 123534).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the local human research ethics board at each participating site for CALSNIC.
Informed consent
Written informed consent was obtained from all participants in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1125 kb)
Rights and permissions
About this article
Cite this article
Chenji, S., Ishaque, A., Mah, D. et al. Neuroanatomical associations of the Edinburgh cognitive and Behavioural ALS screen (ECAS). Brain Imaging and Behavior 15, 1641–1654 (2021). https://doi.org/10.1007/s11682-020-00359-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00359-7