Abstract
Objective
To investigate the effects of gambogic acid (GA) on the proliferation and apoptosis of Human lung adenocarcinoma A549 cells in vitro, as well as the regulation of steroid receptor coactivator-3 (SRC-3) to explore the relationship between them.
Methods
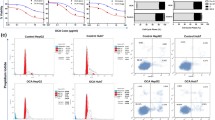
The effect of GA on the growth of A549 cells was studied by MTT assay. Apoptosis was detected through Hoechst 33258 staining. RT-PCR and Western blot technologies were applied to assess the expression of SRC-3, whereas, the localization of SRC-3 was determined by using confocal microscopy method.
Results
GA presented striking proliferation inhibition potency on A549 cells in vitro in a time- and dose-dependent manner, with the IC50 value for 24 h was 3.17±0.13 μmol/L. Hoechst 33258 staining showed that GA could induce apoptosis in A549 cells. Over-expression of SRC-3 was found in A549 cells, whereas the mRNA and protein expression levels of SRC-3 were significantly downregulated in A549 cells induced by GA in a dose-dependent manner. The disposition of SRC-3 was situated mainly at the nuclear.
Conclusion
GA may exert its strong anti-leukemia effects through the regulation of the expression of SRC-3. It may be a new target for the therapy of lung cancer.
Similar content being viewed by others
References
Auterhoff H, Frauendorf H, Liesenklas W, et al. The chief constituent of gamboge resin. 1. Chemistry of gamboge [J]. Arch Pharma 1962; 295:833–846.
Ollis WD, Ramsay MVJ, Sutherland IO, et al. The constitution of gambogic acid [J]. Tetrahedron 1965; 21:1453–1470.
Zhou HJ, Yan J, Luo W, et al. SRC-3 is required for prostate cancer cell proliferation and survival [J]. Cancer Res 2005; 65: 7976–7983.
Zhao L, Guo QL, You QD, et al. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells [J]. Biol Pharm Bull 2004; 27:998–1003.
Guo QL, Lin SS, You QD, et al. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells [J]. Life Sci 2006; 78: 1238–1245.
Wang Y, Chen Y, Chen Z, et al. Gambogic acid induces death inducer-obliterator 1-mediated apoptosis in Jurkat T cells [J]. Acta Pharmacol Sin 2008; 29:349–354.
Anzick SL, Kononen J, Walker RL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer [J]. Science 1997; 277:965–968.
Han SJ, Demayo FJ, Xu J, et al. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor [J]. Mol Endocrinol 2006; 20: 45–55.
Ying H, Furuya F, Willingham MC, et al. Dual functions of the steroid hormone receptor coactivator 3 in modulating resistance to thyroid hormone [J]. Mol Cell Biol 2005; 25:7687–7695.
Lee SK, Kim HJ, Na SY, et al. Steroid receptor coactivator-1 coactivates activating protein-1- mediated transactivations through interaction with the c-Jun and c-Fos subunits [J]. J Biol Chem 1998; 273:16651–16614.
Werbajh S, Nojek I, Lanz R, et al. RAC-3 is a NF-kappa B coactivator [J]. FEBS Lett 2000; 485:195–199.
Chen D, Ma H, Hong H, et al. Regulation of transcription by a protein methyltransferase [J]. Science 1999; 284:2174–2177.
McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators [J]. Cell 2002; 108:465–474.
Kuang SQ, Liao L, Zhang H, et al. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-rasinduced breast cancer initiation and progression in mice [J]. Cancer Res 2004; 64:1875–1885.
Tanner MM, Grenman S, Koul A, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer [J]. Clin Cancer Res 2000; 6:1833–1839.
Henke RT, Haddad BR, Kim SE, et al. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma [J]. Clin Cancer Res 2004; 10:6134–6142.
Ghadimi BM, Schröck E, Walker RL, et al. Specific chromosomal aberrations and amplification of the AIB1 nuclear receptor coactivator gene in pancreatic carcinomas [J]. Am J Pathol 1999; 154:525–536.
Sakakura C, Hagiwara A, Yasuoka R, et al. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers [J]. Int J Cancer 2000; 89:217–223.
Wang Y, Wu MC, Sham JS, et al. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray [J]. Cancer 2002; 95:2346–2352.
Torres-Arzayus MI, Font de Mora J, Yuan J, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene [J]. Cancer Cell 2004; 6:263–74.
Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer[J]. Steroids 2007; 72:135–143.
Lahusen T, Fereshteh M, Oh A, et al. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1 [J]. Cancer Res 2007; 67: 7256–7265.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (No. 30472267).
Rights and permissions
About this article
Cite this article
Li, R., Chen, Y., Shu, Wx. et al. Effects of gambogic acid on regulation of steroid receptor coactivator-3 in lung adenocarcinoma A549 cells. Chin. J. Cancer Res. 21, 68–73 (2009). https://doi.org/10.1007/s11670-009-0068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11670-009-0068-x