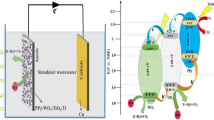
Abstract
In order to enhance the photoelectric conversion efficiency of a photoanode and broaden its light absorption range, a ZnIn2S4/UiO-66/TiO2/Ti composite photoanode was developed. The maximum photocurrent density of the prepared photoanode is 2.56 mA cm−2 at 1.2 V (versus SCE), which is 1.79 times higher than that of ZnIn2S4/TiO2/Ti. The rhodamine B (RhB) removal rate, short-circuit current density and maximum power density of a photocatalytic fuel cell (PFC) constructed with the ZnIn2S4/UiO-66/TiO2/Ti photoanode and a Cu cathode are 98.01%, 0.22 mA cm−2 and 16.97 μW cm−2 respectively. The enhancement of the PFC performance is mainly attributed to the extremely good energy band matching and nano-size interface contact between ZnIn2S4 and UiO-66. The existence of UiO-66 can be as an electronic transmission bridge, which not only facilitates the rapid transfer and utilization of photogenerated electrons, but also improves the adsorption ability of the photoanode for RhB molecules.
Graphical Abstract

Similar content being viewed by others
Introduction
Nowadays, with the acceleration of urbanization and industrialization, the energy crisis and environmental pollution have become increasingly prominent. In particular, a large amount of industrial wastewater and organic pollutants are discharged into natural river systems and artificial water channels, which not only wastes the chemical energy of the organic pollutants, but also causes serious water pollution.1,2 As a revolutionary method, photocatalytic technology has been widely studied and applied in water pollution abatement.3 A photocatalytic fuel cell (PFC) combines photocatalysis (PC) with a fuel cell (FC), which synergizes oxidative degradation of organic pollutants and electrical energy generation. Based on the above advantages, the PFC has great potential in coping with the problems of environmental pollution and energy shortage.3,4,5 In the traditional PFC process, carriers (e−–h+) are generated at the photoanode under light illumination, then the photogenerated electrons can be transferred from the photoanode to the cathode via an external circuit under the action of the potential difference, and the photogenerated holes are involved in the oxidation of organic pollutants.6,7,8 The photoanode plays an essential role in the PFC system; thus it is key to designing an effective and stable photoanode to improve the PFC performance.9
TiO2 is often used as the photoanode of PFC due to its outstanding photocatalytic activity and chemical stability.10,11 Unfortunately, TiO2 can only absorb ultraviolet (UV) light and has a high carrier recombination rate,12,13 resulting in low utilization efficiency of solar energy and electrons. Therefore, researchers modified TiO2 through metal and non-metal doping,14,15 semiconductor recombination,16,17,18 morphology control19 and other novel strategies to promote the electron–hole pair separation and prolong its light response wavelength. Among the multitudinous improvement methods, combining TiO2 with other narrow bandgap semiconductors such as g-C3N4,20,21 BiOBr,22 ZnFe2O4,23 BiVO424 and CdS25,26 to construct different types of heterojunctions is an effective strategy to improve its photocatalytic performance. Ternary metal chalcogenides have been extensively investigated due to their unique optoelectronic and catalytic properties.27 ZnIn2S4 is a typical narrow bandgap photocatalyst with excellent visible-light response performance. In comparison with other metal sulfides, ZnIn2S4 possesses the significant advantages of non-toxicity, easy preparation and excellent stability. Additionally, because of its layered structure, ZnIn2S4 is easy to combine with other semiconductor catalysts to construct high-performance photocatalytic systems.28,29 Previous studies have proved that the introduction of ZnIn2S4 on the surface of TiO2 can be an effective strategy for restraining the recombination of electron–hole pairs and improving the visible-light response performance.30,31 However, most of these reports are about the application of a ZnIn2S4/TiO2 composite in photoelectrochemical cells and photoelectrochemical cathodic protection,32,33,34 but few studies involve photocatalytic fuel cells. In addition, a thin-film photoelectrode prepared by stacking or directly coating composite semiconductor nanomaterial is not conducive to electron transfer. Thus, finding an electron transfer medium between ZnIn2S4 and TiO2 to improve their photoelectric conversion efficiency is critical.
Metal–organic frameworks (MOFs) have been extensively researched in the area of photocatalysis in recent years.35 As functional materials, MOFs possess perfect crystalline ordered structure, special photoelectric properties and high specific surface area, which not only provides additional active sites and catalytic substrate transmission channels for the photocatalytic reaction, but also promotes the rapid transfer and utilization of photogenerated charge carriers.36,37 UiO-66 (Zr) is a zirconium-based MOF, which not only has the same excellent properties as other MOF, but also shows better chemical and thermal stability in water,38,39,40 which makes it a promising photocatalyst for water treatment.
In this study, flower-like ZnIn2S4/UiO-66 hybrid material was used to modify TiO2/Ti to obtain the visible-light responsive ZnIn2S4/UiO-66/TiO2/Ti composite photoanode. The PFC system was constructed with ZnIn2S4/UiO-66/TiO2/Ti photoanode and Cu cathode. Rhodamine B (RhB) was chosen as the simulated wastewater dye to investigate the pollutant removal efficiency and electricity generation capacity of the PFC. Finally, the photocatalytic enhancement mechanism of the photoanode was proposed.
Experimental
Materials
Zirconium chloride (ZrCl4), zinc chloride (ZnCl2) and 1,4-benzene dicarboxylic acid (H2BDC) were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Tetrabutyl titanate (C16H36O4Ti), N,N-dimethylformamide (DMF), sodium sulphate (Na2SO4), ethyl alcohol absolute (CH3CH2OH), acetic acid (CH3COOH), ethylenediamine tetraacetic acid disodium salt (EDTA-2Na), thioacetamide (TAA), rhodamine B (RhB) and isopropyl alcohol (IPA) were purchased from Chron Chemicals Co., Ltd. (Chengdu, China). Indium chloride tetrahydrate (InCl3·4H2O) was purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Ti and Cu were supplied by Hongtai Metal Products Co., Ltd. (Shanghai, China).
Preparation of the Photoelectrode
Synthesis of TiO2 Sol–gel
TiO2 sol–gel was prepared by controlling the hydrolysis of tetrabutyl titanate in ethanol solvent. Specifically, 27 mL tetrabutyl titanate was fully dispersed in 23 mL anhydrous ethanol and the mixed solution was named solution A. Solution B was prepared by adding 2.7 mL deionized water and 3.3 mL hydrochloric acid to 23 mL absolute ethanol. After that, solution B was added dropwise to solution A under continuous stirring, and the mixture was further stirred for 2 h, after which the obtained colloidal solution was placed in the dark for 24 h.
Synthesis of UiO-66
In a typical synthesis, 1 mmol ZrCl4 and 1 mmol H2BDC were dispersed in 60 mL DMF by sonication for 20 min. After that, 1 mL acetic acid was added into the mixture to control the size of UiO-66 nanoparticles and the mixture was stirred for 10 min. Then, the transparent solution was placed into a Teflon-lined autoclave which was subsequently heated to 120°C and kept for 24 h. The acquired white product was washed three times with DMF, and finally soaked in methanol for 3 days to remove DMF solvent.
Synthesis of ZnIn2S4/UiO-66 Composite
ZnIn2S4/UiO-66 composite was prepared by a solvothermal method. First, a certain amount (10.58 mg, 21.16 mg, 31.74 mg) of UiO-66 was added into 30 mL ethanol solution (VH2O:Vethanol = 2:1) and the mixture was sonicated for 30 min to obtain a uniform suspension. Subsequently, 0.25 mmol ZnCl2, 0.5 mmol InCl3·4H2O and 1.5 mmol TAA were dissolved in the above solution and stirred for 20 min. The resultant mixture was transferred into a Teflon-lined autoclave and kept at 160°C for 6 h. After reaction, the final product was centrifuged and washed with deionized water and ethanol 2–3 times, and then dried at 60°C. The obtained nanocomposite material was denoted as Z@U-X (X = 10, 20 or 30). For comparison, pristine ZnIn2S4 was also synthesized by the same procedure without adding UiO-66.
Preparation of the ZnIn2S4/UiO-66/TiO2/Ti Photoanode
The main preparation process of the ZnIn2S4/UiO-66/TiO2/Ti photoanode is shown in Scheme 1. First, a Ti sheet was cut into pieces of the same size (3 × 5 cm2), polished with sandpaper, and cleaned ultrasonically with ethanol and deionized water, respectively. Second, the sheet was coated with the as-prepared TiO2 sol–gel by dip-coating and aged in an oven at 120°C. After repeated coating three times, the precursor electrode was transferred to a high-temperature tubular furnace, heated to 550°C and kept for 2 h to obtained anatase TiO2. Third, 200 mg ZnIn2S4/UiO-66 composite was added into 5 mL ethanol to get ZnIn2S4/UiO-66 suspension liquid, and then the mixture was loaded on the TiO2/Ti electrode by drop-coating. After repeated coating three times, the acquired photoanode was annealed at 140°C for 2 h in a nitrogen atmosphere to strengthen adhesion. According to the quality of the TiO2/Ti electrode before and after loading ZnIn2S4/UiO-66, the total loading quality of ZnIn2S4/UiO-66 composite was calculated to be about 18 mg. The obtained ZnIn2S4/UiO-66/TiO2/Ti composite photoanode with different UiO-66 content was denoted as Z@U-X/TiO2/Ti (X = 10, 20 or 30). For comparison, a ZnIn2S4/TiO2/Ti photoanode was obtained by the same preparation procedure of Z@U-X/TiO2/Ti electrode, except that pure ZnIn2S4 was used instead of ZnIn2S4/UiO-66 composite. According to the quality of TiO2/Ti electrode before and after loading ZnIn2S4 and ZnIn2S4/UiO-66, and the percentage of ZnIn2S4 and UiO-66 in ZnIn2S4/UiO-66 composite, the quality of ZnIn2S4 and TiO2 in both ZnIn2S4/TiO2/Ti and Z@U-20/TiO2/Ti electrodes was calculated to be about 14 mg and 16 mg, respectively. The TiO2/Ti photoanode was acquired by the first and second preparation steps of the Z@U-X/TiO2/Ti electrode.
Photocatalytic Experiments
In the experiments, a cubic quartz pond was used as the photocatalytic reactor, and the as-prepared photoanode was placed upright in the reactor, with the photocatalyst film facing the light source. Similarly, the Cu cathode was fixed in parallel behind the photoanode and they were connected by an external wire. RhB solution was circulated by a peristaltic pump at a speed of 160 mL min−1 above the reactor to enhance mass transfer capacity. The photoanode was illuminated by a 500 W Xe lamp (Shanghai Lansheng) with a filter to produce a light wavelength below 420 nm, and the distance between the light source and the photoanode was about 25 cm. The photoelectrochemical performance of the photoanode in the PFC was explored through a typical three-electrode system, in which a calomel electrode (SCE), a platinum electrode (Pt) and the as-prepared photoanode were used as the reference electrode, the counter electrode and the working electrode, respectively. The RhB degradation ability of the PFC was evaluated by a two-electrode system with the following conditions: 10 mg L−1 RhB solution (125 mL), initial solution pH 3.0 (adjusted with 0.5 M H2SO4 or NaOH), and 0.1 mol L−1 Na2SO4 as electrolyte. During the RhB degradation experiment, about 3 mL sample was taken every 10 min to analyze the concentration of RhB.
Characterization and Analysis Methods
X-ray diffractometry (XRD; XRD-7000X, Shimadzu) was employed to study the phases of different samples. The morphology of as-prepared photoanodes was observed by scanning electron microscopy (SEM; Sigma500, Germany). Fourier transform infrared spectroscopy (FT-IR; Thermo Scientific Nicolet 380, USA) was used to investigate the molecular structure of ZnIn2S4/UiO-66/TiO2 hybrid material. The valence states of different elements were analyzed by X-ray photoelectron spectroscopy (XPS; Thermo Scientific K-Alpha, USA). UV–vis diffuse reflectance spectroscopy (DRS; UV2600, Japan) was employed to measure the optical properties of different materials. Ultraviolet–visible spectrophotometry (UV6100, METASH, Shanghai, China) was used to measure the concentration change of RhB solution at its maximum wavelength of 554 nm. An electrochemical workstation (CHI650E, Beijing Huake Putian Technology Co., Ltd.) was used to measure and record the linear sweep voltammograms (LSV) of the photoanode, photocurrent density–voltage (J–V) and photocurrent response curve of the PFC.
Results and Discussion
Characterization of the Photoelectrode
The XRD diffractograms of pure UiO-66, ZnIn2S4 and TiO2/Ti, ZnIn2S4/TiO2/Ti, Z@U-20/TiO2/Ti electrodes are described in Fig. 1. The characteristic peak of UiO-66 can be attributed to the crystallographic UiO-66 phase and is consistent with that reported in the literature.41,42 For pure ZnIn2S4, the diffraction peaks at 21.4°, 27.5°, 30.4°, 47.2°, 52.3° and 55.6° correspond to (006), (102), (104), (110), (116) and (202) planes of hexagonal ZnIn2S4 (JCPDS No. 65-2023),28 respectively. The TiO2/Ti electrode exhibits diffraction peaks at 25.2° (101), 38.6° (112), 48.0° (200), 53.8° (105) and 55.0° (211), which demonstrates that anatase TiO2 is successfully prepared (JCPDS No. 21-1272). For the Z@U-20/TiO2/Ti electrode, the characteristic diffraction peaks belonging to ZnIn2S4 and UiO-66 can be obviously found, indicating that TiO2/Ti has been successfully modified by ZnIn2S4/UiO-66 composite.
FT-IR spectra were further analyzed to investigate the structural integrity of UiO-66 in the hybrid material (Fig. 2). For pure UiO-66, the broad peak at approximately 3410 cm−1 can be attributed to the O-H bending vibration,43 and the absorption peaks at 1578 and 1398 cm−1 correspond to the O-C-O asymmetric and symmetric stretching vibration, respectively.38 The peak at 1509 cm−1 represents the C=C stretching of the benzene ring.44 All the characteristic peaks of UiO-66 remained intact in the composite, proving the successful introduction of UiO-66. The strong peak of ZnIn2S4/UiO-66/TiO2 at about 510 cm−1 is the result of the Ti-O tensile vibration.45 Furthermore, the weak peak at 1654 cm−1 is ascribed to the C=O asymmetric stretching vibration of DMF,46 which disappears in the spectrum of ZnIn2S4/UiO-66/TiO2, suggesting that DMF molecules in the pores of UiO-66 can be effectively removed after soaking in methanol.
Figure 3 exhibits the SEM images of TiO2/Ti and Z@U-20/TiO2/Ti photoelectrodes. The pristine TiO2/Ti electrode is formed by the ordered arrangement of fine TiO2 nanoparticles, and presents a porous structure, which provides perfect conditions for loading other catalysts (Fig. 3a). In Fig. 3b, ZnIn2S4 shows flower-like microsphere structure composed of numerous nanosheets, and the UiO-66 particles are uniformly dispersed into the nanosheets of ZnIn2S4. ZnIn2S4/UiO-66 microspheres can be clearly observed on the surface of TiO2, indicating that the composite photoelectrode Z@U-20/TiO2/Ti has been successfully prepared, which is consistent with the results of the XRD pattern and FT-IR spectrum observation. The surface design of photoanode has always been an important strategy to enhance the properties of a PFC. It is noteworthy that the surface of the flower-like ZnIn2S4/UiO-66 nanocomposite modified TiO2 electrode is rougher than that of TiO2/Ti, which enables it to have larger reactant contact area and light exposure area.
To further analyze the valence states of elements in ZnIn2S4/UiO-66/TiO2 composite, XPS measurements were conducted. It can be observed from the survey XPS spectrum (Fig. 4a) that the ZnIn2S4/UiO-66/TiO2 hybrid material consists of Zn, O, Ti, In, C, Zr and S. In Fig. 4b, there are two peaks at 464.4 eV and 458.7 eV, which are attributed to Ti 2p1/2 and Ti 2p3/2, respectively, and the splitting energy is 5.7 eV, which matches well with the data of Ti4+.47 The Zn 2p XPS spectrum can be split into Zn 2p3/2 (1021.9 eV) and Zn 2p1/2 (1045 eV) peaks (Fig. 4c), which correspond to Zn2+.30 Figure 4d exhibits two peaks at 182.8 eV and 185.1 eV, which belong to Zr 2p5/2 and Zr 2p3/2, respectively, indicating that Zr exists as Zr6+ in the zirconium-oxo cluster.48 Two peaks at 445.1 eV and 452.6 eV are, respectively, ascribed to In 3d5/2 and In 3d3/2 of In3+ (Fig. 4e).49 The S 2p XPS spectrum (Fig. 4f) can be fitted into two main peaks locating at 161.8 eV and 162.9 eV, which belong to S 2p3/2 and S 2p1/2, respectively.39 The O 1s XPS spectrum (Fig. 4g) includes three peaks at 529.9 eV, 531.1 eV and 532.3 eV, which correspond to surface -OH, C=O and Zr-O, respectively.50 In Fig. 4h, the peaks at 288.8 eV, 286.3 eV and 284.8 eV are attributed to the C=O of the carboxylic group, C-C and C=C of benzene rings, respectively. The above results demonstrate that ZnIn2S4/UiO-66/TiO2/Ti photoanode has been successfully prepared.
The optical properties of pure ZnIn2S4, TiO2/Ti and Z@U-20/TiO2/Ti electrodes were evaluated by UV–vis DRS. As shown in Fig. 5a, the light absorption range of TiO2/Ti is restricted to the ultraviolet region, while in contrast, ZnIn2S4 and Z@U-20/TiO2/Ti exhibit excellent absorption performance in the visible-light region, and their absorption edges are significantly redshifted. The light absorption edges of different samples were acquired by processing their DRS. The absorption edges of TiO2/Ti, Z@U-20/TiO2/Ti and ZnIn2S4 are 403 nm, 540 nm and 554 nm, respectively. According to Eq. 1,51 the calculated bandgap energies are 3.08 eV, 2.30 eV and 2.24 eV for TiO2/Ti, Z@U-20/TiO2/Ti and ZnIn2S4, respectively. In order to further discuss the bandgap nature of Z@U-20/TiO2/Ti, the bandgap energy of the Z@U-20/TiO2/Ti electrode was also calculated by Tauc's equation (Eq. 2).48
where α, h, v, A and Eg represent the absorption coefficient, Planck constant, the light frequency, proportional constant and bandgap energy, respectively. The values of n are 2 and 1/2 for direct transition and indirect transition, respectively. According to Eq. 2, the calculated bandgap energies of the Z@U-20/TiO2/Ti electrode are 2.30 and 2.00 eV by taking n = 2 and n = 1/2 (Fig. 5b), respectively. It can be found that when n is 2, Eg (2.30 eV) calculated by the Tauc equation is highly consistent with the result of DRS, indicating that Z@U-20/TiO2/Ti possesses a direct bandgap. The above experimental results show that the introduction of ZnIn2S4/UiO-66 composite on the surface of TiO2/Ti can extend its light response range to the visible-light region, which means that the Z@U-20/TiO2/Ti electrode can absorb more photons and more photoexcited carriers can be generated. This creates favorable conditions for the improvement of photocatalytic performance.
RhB Degradation Performance of PFC
In this experiment, RhB was chosen as the simulated wastewater dye to evaluate the degradation performance of PFCs with a Cu cathode and different photoanodes, including TiO2/Ti-Cu (named Ti-PFC), ZnIn2S4/TiO2/Ti-Cu (named ZIS/Ti-PFC) and Z@U-X/TiO2/Ti-Cu (named Z@U-X/Ti-PFC, X = 10, 20 or 30). The results are shown in Fig. 6a. RhB is relatively stable under visible-light irradiation, and Ti-PFC shows relatively much lower RhB degradation ability, while the degradation rate of RhB in ZIS/Ti-PFC (81.3%) is much higher than that of Ti-PFC (33.4%) due to the excellent visible-light absorption property of ZnIn2S4/TiO2/Ti photoanode after the introduction of ZnIn2S4 on the surface of TiO2. Moreover, all the three Z@U-X/Ti-PFCs possess significantly higher RhB degradation rates than ZIS/Ti-PFC and Ti-PFC, and Z@U-20/Ti-PFC has an optimal RhB degradation rate (98.1%), which demonstrates that the presence of UiO-66 has a positive impact on RhB degradation.
It is noteworthy that with the increase of UiO-66 content, the degradation rate increases firstly and then decreases. This means that the doping amount of UiO-66 is a critical factor affecting the photocatalytic activity. When the doping amount of UiO-66 is too low (for example, X = 10), the assistance for electron transfer is very limited. However, the excessive UiO-66 (for example, X = 30) may agglomerate on the surface of ZnIn2S4 nanosheets and affect the absorption of visible-light by the active component ZnIn2S4.38 An appropriate amount UiO-66 (for example, X = 20) forms an intimate interface contact with ZnIn2S4, which facilitates the transfer of photogenerated electrons from ZnIn2S4 to UiO-66, thus inhibiting the recombination of photoexcited carriers. Meanwhile, the presence of UiO-66 also enhances the adsorption ability of the photoanode and provides additional active reaction sites for RhB degradation. Thus, the photocatalytic degradation activity of Z@U-X/Ti-PFC has been enhanced.
To compare the degradation efficiency of different PFCs more intuitively, the pseudo-first-order kinetic model (Eq. 3) was employed to fit the degradation data of the first hour (Fig. 6b). It can be seen that Z@U-20/Ti-PFC exhibits the highest degradation rate among all PFCs. Its kinetic rate constant k is 0.0410 min−1, which is 7.23 and 2.43 times higher than that of Ti-PFC (0.0057 min−1) and ZIS/Ti-PFC (0.0168 min−1), respectively.
where C0 and Ct represent the concentration (mg L−1) of RhB at degradation time 0 and t (min), respectively.39
To elucidate the photocatalytic reaction mechanism of RhB degradation by the Z@U-20/Ti-PFC system, free radical trapping experiments were conducted. IPA, EDTA-2Na and N2 purging were chosen to capture ·OH, h+ and ·O2− respectively. The results are illustrated in Fig. 6c. When IPA is added during the photocatalytic reaction, the degradation rate of RhB is not affected distinctly. However, the addition of EDTA-2Na and N2 can obviously inhibit the degradation efficiency of RhB, which decreases from 98.1% to 79.6% and 38.9% respectively. The above results suggest that h+ and ·O2− participated in the removal of RhB, and ·O2− plays a crucial role in the degradation process.
Figure 6d presents the change of UV–vis absorption spectrum of RhB solution with reaction time. It can be clearly found that the maximum absorption peak gradually decreases and shifts to the blue region with the progress of the reaction, which proves that deethylation and chromophore breakage occur concurrently.52 Because the falling speed of the maximum absorption peak is much faster than the blueshift, it is considered that the chromophore group breakage is the main method of RhB degradation.
Electricity Generation Performance of PFC
LSV is an advantageous tool to measure the photocurrent density and surface charge transfer. The LSV curves of different photoelectrodes under visible-light irradiation with the potential range from −0.6 V to 1.2 V (versus SCE) are depicted in Fig. 7a. The TiO2/Ti electrode presents a relatively weak photocurrent density (0.02 mA cm−2 at 1.2 V (versus SCE)), which can be indexed to the narrow light absorption range and low quantum efficiency of pristine TiO2. After introducing ZnIn2S4 on the surface of TiO2, the photocurrent density of the ZnIn2S4/TiO2/Ti photoanode increases significantly to 1.42 mA cm−2 at 1.2 V (versus SCE) because of the expansion of light response range, thus resulting in more photogenerated electrons under light irradiation. In addition, all three Z@U-X/TiO2/Ti (X = 10, 20, 30) electrodes display much higher photocurrent density in comparison to TiO2/Ti and ZnIn2S4/TiO2 electrodes, which is consistent with the results of degradation experiments (Fig. 6a), once again proving that the good energy band matching makes UiO-66 the bridge of charge transfer between ZnIn2S4 and TiO2. The synergistic effect among different components enables electrons to be quickly transferred and utilized and finally enhances the photoelectric conversion efficiency. The Z@U-20/TiO2/Ti electrode obtains the highest photocurrent density (2.56 Ma cm−2 at 1.2 V (versus SCE)), which is 1.79 times that of ZnIn2S4/TiO2/Ti. The J–V characteristics and power density of different PFCs are shown in Fig. 7b. Certainly, the Z@U-20/Ti-PFC obtains optimal power generation performance, which is consistent with the results of the LSV curve (Fig. 7a). Its maximum power density (Pmax) and short-circuit current density (Jsc) were 16.97 μW cm−2 and 0.22 mA cm−2, respectively.
The transient photocurrent response and reusability test of Z@U-20/Ti-PFC are shown in Fig. 8. It can be found that the photocurrent decreases rapidly and approaches zero when the light is turned off, and recovers when the light is turned on again. This result means that the photoanode has an excellent visible-light response performance (Fig. 8a). As seen in Fig. 8b, high degradation performance of Z@U-20/Ti-PFC is maintained with only 8.9% loss in degradation efficiency observed after five cycling experiments, which confirms its outstanding reusability. The slight decrease of degradation activity may be because some of the active reaction sites on the surface of the catalyst are occupied by RhB molecules in the process of repeated experiments.
In order to further assess the performance of Z@U-20/Ti-PFC, it is compared with other PFCs in the literature. As illustrated in Table I, the PFC system in this work exhibits superior pollutant degradation performance, short-circuit current density (Jsc) and maximum power density (Pmax).53,54,55,56,57,58,59,60 By comparing with our previous study,55,59 it can be found that the increased surface roughness of the photoanode has a positive effect on the degradation of pollutants. In addition, the PFC system with a Cu2O photocathode has better power generation ability than that with a Cu cathode. This may be because as a p-type semiconductor, the Fermi level of Cu2O is close to the valence band, and the difference of Fermi level between two photoelectrodes can induce electron transfer from the photoanode to the photocathode though the external circuit,8 thus showing enhanced photoelectric conversion efficiency.
Mechanism of Z@U/Ti-PFC
The above experimental results prove that after the introduction of the flower-like ZnIn2S4/UiO-66 nanocomposite on the TiO2 surface, the pollutant removal capacity and power generation performance of the Z@U/Ti-PFC system constructed by the ternary composite photoanode are significantly improved, which can be attributed to the good visible-light response of ZnIn2S4 and the synergistic effect among different materials to realize the rapid transfer of charge (Scheme 2). It is well known that the band edge position of different semiconductor materials has an important impact on their photocatalytic properties.61 To further study the RhB degradation mechanism and electron transfer process of Z@U-20/TiO2/Ti photoanode, the band edge potential of valence band (VB) and conduction band (CB) were estimated by Eqs. 4 and 5.62
where χ is the electronegativity, Ee is the energy of free electrons on the hydrogen scale (4.5 eV) and Eg is the bandgap of the semiconductor. For pure TiO2 and ZnIn2S4, the χ values are 4.82 eV63 and 5.81 eV,64 respectively. The Eg of TiO2 and ZnIn2S4 are 3.08 eV and 2.24 eV (refer to the results of DRS), respectively. Thus, ECB and EVB of TiO2 are estimated to be −0.23 eV and 2.85 eV, respectively, and the corresponding values of ZnIn2S4 are about −0.80 eV and 1.44 eV, respectively. Moreover, the application of MOFs in the area of photocatalysis is due to its semiconductor-like properties, and the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) gaps of the connecting molecules are the major determinants of MOF bandgap.65 According to literature reports,41,42 the LUMO energy of UiO-66(Zr) is about −0.6 eV (versus NHE), and the HOMO energy is about 3.3 eV (versus NHE). Based on the above results, the enhanced mechanism diagram of the synergistic effect of TiO2, ZnIn2S4 and UiO-66 is proposed. First, as the visible-light active component, the electrons in VB of ZnIn2S4 are excited to CB under visible-light irradiation, leaving holes in its VB simultaneously. Since the CB potential of ZnIn2S4 (−0.80 eV) is more negative than the LUMO of UiO-66 (−0.6 eV), photoelectrons can be quickly transferred from the CB of ZnIn2S4 to the LUMO of UiO-66 under the action of potential difference, and the VB potential (1.44 eV) is less positive than (H2O/·OH) (2.27 eV); thus, ·OH cannot be produced. Meanwhile, the LUMO energy of UiO-66 is more negative compared to (O2/·O2−) (−0.33 eV), which means that the photoelectrons at the LUMO of UiO-66 can be captured by dissolved O2 to produce ·O2−, which together with holes in VB of ZnIn2S4 participate in the oxidation reaction of RhB molecules. Further, the electrons in the LUMO level of UiO-66 can also be moved to the CB of TiO2 and then transmitted to the cathode via an external electric field to generate electric energy. The above mechanism analysis was verified by the experimental results of radical quenching in Fig. 6c.
Conclusion
In this paper, a ZnIn2S4/UiO-66/TiO2/Ti composite photoanode was successfully prepared through a facile coating method and applied for energy recovery and organic pollutant degradation in a PFC system. The prepared photoanode exhibits unique structure and photoelectric properties, including rough surface, well-matched energy band, strong absorption in the visible area, rapid carrier migration and good stability. Based on the synergistic effect among different materials, the rapid transfer and utilization of photogenerated electrons can be realized, thus simultaneously improving the photoelectric conversion efficiency and pollutant degradation ability of PFC. The RhB removal efficiency, photocurrent density and maximum power density of the optimal Z@U-20/Ti-PFC were 98.01%, 0.22 mA cm−2 and 16.97 μW cm−2, respectively. This work provides a reference for the design of an efficient thin-film photoanode.
References
J.C.G. Sousa, A.R. Ribeiro, M.O. Barbosa, M.F.R. Pereira, and A.M.T. Silva, A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. J. Hazard. Mater. 344, 146 (2018).
R.A. Sheldon, Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 6, 32 (2018).
G. Ren, H. Han, Y. Wang, S. Liu, J. Zhao, X. Meng, and Z. Li, Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 11, 1804 (2021).
P. Mishra, P. Saravanan, G. Packirisamy, M. Jang, and C. Wang, A Subtle Review on the Challenges of Photocatalytic Fuel Cell for Sustainable Power Production. Int. J. Hydrogen Energy 46, 22877 (2021).
Y. Vasseghian, A. Khataee, E.N. Dragoi, M. Moradi, S. Nabavifard, G.O. Conti, and A.M. Khaneghah, Pollutants Degradation and Power Generation by Photocatalytic Fuel Cells: A Comprehensive Review. Arab. J. Chem. 13, 8458 (2020).
B. Li, Y. He, M. Xiao, Y. Zhang, Z. Wang, Z. Qin, B. Chai, J. Yan, J. Li, J. Li, and Z. Cao, A Solar-Light Driven Photocatalytic Fuel Cell for Efficient Electricity Generation and Organic Wastewater Degradation. Colloids Surf. A 642, 128205 (2022).
M. Li, Y. Liu, L. Dong, C. Shen, F. Li, M. Huang, C. Ma, B. Yang, X. An, and W. Sand, Recent Advances on Photocatalytic Fuel Cell for Environmental Applications—The Marriage of Photocatalysis and Fuel Cells. Sci. Total Environ. 668, 966 (2019).
Y. He, K. Chen, M.K.H. Leung, Y. Zhang, L. Li, G. Li, J. Xuan, and J. Li, Photocatalytic Fuel Cell—A Review. Chem. Eng. J. 428, 131074 (2022).
L. Zha, J. Bai, C. Zhou, Y. Zhang, J. Li, P. Wang, B. Zhang, and B. Zhou, Treatment of Hazardous Organic Amine Wastewater and Simultaneous Electricity Generation Using Photocatalytic Fuel Cell Based on TiO2/WO3 Photoanode and Cu Nanowires Cathode. Chemosphere 289, 133119 (2022).
P. Mikrut, D. Mitoraj, R. Beranek, and W. Macyk, Facet-Dependent Activity of Tailored Anatase TiO2 Crystals in Photoanodes for Photocatalytic Fuel Cells. Appl. Surf. Sci. 566, 150662 (2021).
Y. Ye, H. Bruning, X. Li, D. Yntema, and H.H.M. Rijnaarts, Significant Enhancement of Micropollutant Photocatalytic Degradation Using a TiO2 Nanotube Array Photoanode Based Photocatalytic Fuel Cell. Chem. Eng. J. 354, 553 (2018).
A. Purabgola, N. Mayilswamy, and B. Kandasubramanian, Graphene-Based TiO2 Composites for Photocatalysis & Environmental Remediation: Synthesis and Progress. Environ. Sci. Pollut. Res. 29, 32305 (2022).
W.A. Zoubi, A.A.S.A. Hamdani, B. Sunghun, and Y.G. Ko, A Review on TiO2-Based Composites for Superior Photocatalytic Activity. Rev. Inorg. Chem. 41, 213 (2021).
P. Gao, L. Yang, S. Xiao, L. Wang, W. Guo, and J. Lu, Effect of Ru, Rh, Mo, and Pd Adsorption on the Electronic and Optical Properties of Anatase TiO2(101): A DFT Investigation. Materials 12, 814 (2019).
Y. Guo, T. Guo, J. Chen, J. Wei, L. Bai, X. Ye, Z. Ding, W. Xu, and Z. Zhou, Synthesis of C-N–S co-Doped TiO2 Mischcrystal with an Isobandgap Characteristic and its Photocatalytic Activity Under Visible Light. Catal. Sci. Technol. 8, 4108 (2018).
M. Zalfani, Z.Y. Hu, W.B. Yu, M. Mahdouani, R. Bourguiga, M. Wu, Y. Li, G.V. Tendeloo, Y. Djaoued, and B.L. Su, BiVO4/3DOM TiO2 Nanocomposites: Effect of BiVO4 as Highly Efficient Visible Light Sensitizer for Highly Improved Visible Light Photocatalytic Activity in the Degradation of Dye Pollutants. Appl. Catal. B Environ. 205, 121 (2017).
Q. Liu, D. Zhai, Z. Xiao, C. Tang, Q. Sun, C.R. Bowen, H. Luo, and D. Zhang, Piezo-Photoelectronic Coupling Effect of BaTiO3@TiO2 Nanowires for Highly Concentrated Dye Degradation. Nano Energy 92, 106702 (2022).
X. Cheng, R. Guan, Y. Chen, Y. Sun, and Q. Shang, The Unique TiO2(B)/BiOCl07I03-P Z-Scheme Heterojunction Effectively Degrades and Mineralizes the Herbicide Fomesafen. Chem. Eng. J. 431, 134021 (2022).
L. Xiang and X. Zhao, Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 7, 310 (2017).
C. Xu, D. Li, X. Liu, R. Ma, N. Sakai, Y. Yang, S. Lin, J. Yang, H. Pan, J. Huang, and T. Sasaki, Direct Z-Scheme Construction of g-C3N4 QUANTUM dots/TiO2 Nanoflakes for Efficient Photocatalysis. Chem. Eng. J. 430, 132861 (2022).
J. Wang, G. Wang, B. Cheng, J. Yu, and J. Fan, Sulfur-Doped g-C3N4/TiO2 S-Scheme Heterojunction Photocatalyst for Congo Red Photodegradation. Chin. J. Catal. 42, 56 (2021).
K. Wang, Y. Zhang, L. Liu, N. Lu, and Z. Zhang, BiOBr Nanosheets-Decorated TiO2 Nanofibers as Hierarchical p–n Heterojunctions Photocatalysts for Pollutant Degradation. J. Mater. Sci. 54, 8426 (2019).
D. Zeng, J. Wang, Y. Xie, Y. Ling, J. Zhao, H. Ye, and T. Chen, TiO2@ZnFe2O4 Heterojunctions for Effecicent Photocatalytic Degradation of Persistent Pollutants and Hydrogen Evolution. Mater. Chem. Phys. 277, 125462 (2022).
Y. Liu, Y. Xu, D. Zhong, N. Zhong, and H. Luo, Core–Shell BiVO4@Polythiophene Co-modified TiO2 as an Efficient Photoanode for Visible-Light Responsive Photocatalytic Fuel Cell. Opt. Mater. 111, 110563 (2021).
C. Feng, L. Zhang, and Z. Cheng, Preparation of Spry-Liked CdS-TiO2 One-Dimensional Composite Nanomaterial and Its Photocatalytic Degradation Efficiency. ChemistrySelect 5, 2142 (2020).
S. Wang, D. Liu, J. Yu, X. Zhang, P. Zhao, Z. Ren, Y. Sun, M. Li, and S. Han, Photocatalytic Penicillin Degradation Performance and the Mechanism of the Fragmented TiO2 Modified by CdS Quantum Dots. ACS Omega 6, 18178 (2021).
G. Yadav and Md. Ahmaruzzaman, ZnIn2S4 and ZnIn2S4 Based Advanced Hybrid Materials: Structure, Morphology and Applications in Environment and Energy. Inorg. Chem. Commun. 138, 109288 (2022).
J. Wang, S. Sun, R. Zhou, Y. Li, Z. He, H. Ding, D. Chen, and W. Ao, A Review: Synthesis, Modification and Photocatalytic Applications of ZnIn2S4. J. Mater. Sci. Technol. 78, 1 (2021).
Y. Pan, X. Yuan, L. Jiang, H. Yu, J. Zhang, H. Wang, R. Guan, and G. Zeng, Recent Advances in Synthesis, Modification and Photocatalytic Applications of Micro/Nano-Structured Zinc Indium Sulfide. Chem. Eng. J. 354, 407 (2018).
G. Song, Q. Zhang, F. Liu, C. Wang, R. Yang, Z. Chen, and D. Ma, Mixed-Phase ZnIn2S4 Nanosheets Grown on TiO2 Nanotrees for the Visible-Light Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 5, 380 (2022).
M. Li, J. Qiu, L. Yang, Y. Feng, and J. Yao, Fabrication of TiO2 Embedded ZnIn2S4 Nanosheets for Efficient Cr(VI) Reduction. Mater. Res. Bull. 122, 110671 (2020).
H. Li, Y. Li, X. Wang, and B. Hou, 3D ZnIn2S4 Nanosheets/TiO2 Nanotubes as Photoanodes for Photocathodic Protection of Q235 CS with High Efficiency Under Visible Light. J. Alloys Compd. 771, 892 (2019).
I.B. Assaker, M. Gannouni, J.B. Naceur, M.A. Almessiere, A.L. Al-Otaibi, T. Ghrib, S. Shen, and R. Chtourou, Electrodeposited ZnIn2S4 Onto TiO2 Thin Films for Semiconductor-Sensitized Photocatalytic and Photoelectrochemical Applications. Appl. Surf. Sci. 351, 927 (2015).
X. Jiang, M. Sun, Z. Chen, J. Jing, G. Lu, and C. Feng, Boosted Photoinduced Cathodic Protection Performance of ZnIn2S4/TiO2 Nanoflowerbush with Efficient Photoelectric Conversion in NaCl Solution. J. Alloys Compd. 876, 160144 (2021).
X. Zhang, J. Wang, X.X. Dong, and Y.K. Lv, Functionalized Metal–Organic Frameworks for Photocatalytic Degradation of Organic Pollutants in Environment. Chemosphere 242, 125144 (2020).
J.A. Harvey, C.J. Pearce, M.G. Hall, E.J. Bruni, J.B. DeCoste, and D.F.S. Gallis, Insights Into the Solvent-Assisted Degradation of Organophosphorus Compounds by a Zr-Based Metal–Organic Framework†. Dalton Trans. 48, 16153 (2019).
X. Chen, X. Peng, L. Jiang, X. Yuan, H. Yu, H. Wang, J. Zhang, and Q. Xia, Recent Advances in Titanium Metal–Organic Frameworks and Their Derived Materials: Features, Fabrication, and Photocatalytic Applications. Chem. Eng. J. 395, 125080 (2020).
J. Ding, Z. Yang, C. He, X. Tong, Y. Li, X. Niu, and H. Zhang, UiO-66(Zr) Coupled with Bi2MoO6 as Photocatalyst for Visible-Light Promoted Dye Degradation. J. Colloid Interface Sci. 497, 126 (2017).
Q. Hu, S. Yin, Y. Chen, B. Wang, M. Li, Y. Ding, J. Di, J. Xia, and H. Li, Construction of MIL-125(Ti)/ZnIn2S4 Composites with Accelerated Interfacial Charge Transfer for Boosting Visible Light Photoreactivity. Colloids Surf. A 585, 124078 (2020).
Z. Man, Y. Meng, X. Lin, X. Dai, L. Wang, and D. Liu, Assembling UiO-66@TiO2 Nanocomposites for Efficient Photocatalytic Degradation of Dimethyl Sulfide. Chem. Eng. J. 431, 133952 (2022).
A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke, and P. Behrens, Modulated Synthesis of Zr-Based Metal–Organic Frameworks: From Nano to Single Crystals. Chem. Eur. J. 17, 6643 (2011).
R. Bariki, D. Majhi, K. Das, A. Behera, and B.G. Mishra, Facile Synthesis and Photocatalytic Efficacy of UiO-66/CdIn2S4 Nanocomposites with Flowerlike 3D-Microspheres Towards Aqueous Phase Decontamination of Triclosan and H2 Evolution. Appl. Catal. B: Environ. 270, 118882 (2020).
J. Tang, T. Zhang, Q. Zhang, Z. Duan, C. Li, D. Hou, Q. Xv, C. Meng, Y. Zhang, and Y. Zhu, In-Situ Growth UiO-66 on Bi2O3 to Fabrication p-p Heterojunction with Enhanced Visible-Light Degradation of Tetracycline. J. Solid State Chem. 302, 122353 (2021).
B. Liu, X. Liu, J. Liu, C. Feng, Z. Li, C. Li, Y. Gong, L. Pan, S. Xu, and C.Q. Sun, Efficient Charge Separation Between UiO-66 and ZnIn2S4 Flowerlike 3D Microspheres for Photoelectronchemical Properties. Appl. Catal. B: Environ. 226, 234 (2018).
Z. Zhao, W. Zhang, X. Shen, T. Muhmood, M. Xia, W. Lei, F. Wang, and M.A. Khan, Preparation of g-C3N4/TiO2/BiVO4 Composite and Its Application in Photocatalytic Degradation of Pollutant from TATB Production Under Visible Light Irradiation. J. Photochem. Photobiol. A: Chem. 358, 246 (2018).
X. Xu, R. Liu, Y. Cui, X. Liang, C. Lei, S. Meng, Y. Ma, Z. Lei, and Z. Yang, PANI/FeUiO-66 Nanohybrids with Enhanced Visible-Light Promoted Photocatalytic Activity for the Selectively Aerobic Oxidation of Aromatic Alcohols. Appl. Catal. B: Environ. 210, 484 (2017).
N.L. Reddy, S. Emin, M. Valant, and M.V. Shankar, Nanostructured Bi2O3@TiO2 Photocatalyst for Enhanced Hydrogen Production. Int. J. Hydrogen Energy 42, 6627 (2017).
X. Zhang, N. Zhang, C. Gan, Y. Liu, L. Chen, C. Zhang, and Y. Fang, Synthesis of In2S3/UiO-66 Hybrid with Enhanced Photocatalytic Activity Towards Methyl Orange and Tetracycline Hydrochloride Degradation Under Visible-Light Irradiation. Mater. Sci. Semicond. Process. 91, 212 (2019).
X. Peng, L. Ye, Y. Ding, L. Yi, C. Zhang, and Z. Wen, Nanohybrid Photocatalysts with ZnIn2S4 Nanosheets Encapsulated UiO-66 Octahedral Nanoparticles for Visible-Light-Driven Hydrogen Generation. Appl. Catal. B Environ. 260, 118152 (2020).
Q. Liang, W. Gao, C. Liu, S. Xu, and Z. Li, A Novel 2D/1D Core–Shell Heterostructures Coupling MOF-Derived Iron Oxides with ZnIn2S4 for Enhanced Photocatalytic Activity. J. Hazard. Mater. 392, 122500 (2020).
Y. Liu, Y. Xu, D. Zhong, H. Yao, Y. Zeng, N. Zhong, and H. Luo, BiVO4@PDA/TiO2/Ti Photoanode with Polydopamine as Electron Transfer Mediator for Efficient Visible-Light Driven Photocatalytic Fuel Cell. Colloids Surf. A 612, 125941 (2021).
J. Yang, W. Liao, Y. Liu, M. Murugananthan, and Y. Zhang, Degradation of Rhodamine B Using a Visible-Light Driven Photocatalytic Fuel Cell. Electrochim. Acta 144, 7 (2014).
S. Xie, K. Ouyang, and Y. Shao, A Solar Responsive Photocatalytic Fuel Cell with a Heterostructured ZnFe2O4/TiO2-NTs Photoanode and an Air-Breathing Cathode. Int. J. Hydrogen Energy 42, 29201 (2017).
S. Xie, K. Ouyang, and X. Ye, A Novel Visible-Light Responsive Photocatalytic Fuel Cell with a Heterostructured BiVO4/WO3 Photoanode and a Pt/C Air-Breathing Cathode. J. Colloid Interface Sci. 532, 758 (2018).
Y. Liu, Y. Xu, D. Zhong, and N. Zhong, Visible-Light Photocatalytic Fuel Cell with BiVO4/UiO-66/TiO2/Ti Photoanode Efficient Degradation of Rhodamine B and Stable Generation of Electricity. Chem. Phys. 542, 111053 (2021).
L. Hu, Y. Liao, D. Xia, Q. Zhang, H. He, J. Yang, Y. Huang, H. Liu, F. Zhang, C. He, and D. Shu, In-Situ Fabrication of AgI-BiOI Nanoflake Arrays Film Photoelectrode for Efficient Wastewater Treatment, Electricity Production and Enhanced Recovery of Copper in Photocatalytic Fuel Cell. Catal. Today 339, 379 (2020).
B. Deng, S. Fu, Y. Zhang, Y. Wang, D. Ma, and S. Dong, Simultaneous Pollutant Degradation and Power Generation in Visible-Light Responsive Photocatalytic Fuel Cell with an Ag-TiO2 Loaded Photoanode. Nano Struct. Nano Objects 15, 167 (2018).
K. Li, H. Zhang, Y. Tang, D. Ying, Y. Xu, Y. Wang, and J. Jia, Photocatalytic Degradation and Electricity Generation in a Rotating Disk Photoelectrochemical Cell Over Hierarchical Structured BiOBr Film. Appl. Catal. B Environ. 164, 82 (2015).
H. Yao, Y. Xu, D. Zhong, Y. Zeng, and N. Zhong, Efficient Rhodamine B Degradation and Stable Electricity Generation Performance of Visible-Light Photocatalytic Fuel Cell with g-C3N4/WO3/TiO2/Ti Photoanode. Ionics 27, 4875 (2021).
M.D. Salman, S.H. Ammar, and R.F. Shafi, Zincoxysulfide Coated Zinc Ferrite Core/Shell Nanocatalyst: An Efficient Photoanode for Single Chamber Photocatalytic Fuel Cell. Nano Struct. Nano Objects 24, 100572 (2020).
F. Stelo, N. Kublik, S. Ullah, and H. Wender, Recent Advances in Bi2MoO6 Based Z-Scheme Heterojunctions for Photocatalytic Degradation of Pollutants. J. Alloys Compd. 829, 154591 (2020).
T. Yu, W. Wu, L. Liu, C. Gao, and T. Yang, Novel Ternary p-ZnIn2S4/rGO/n-g-C3N4 Z-Scheme Nanocatalyst with Enhanced Antibiotic Degradation in a Dark Self-Biased Fuel Cell. Ceram. Int. 46, 9567 (2020).
J. Yan, G. Wu, N. Guan, and L. Li, Nb2O5/TiO2 Heterojunctions: Synthesis Strategy and Photocatalytic Activity. Appl. Catal. B: Environ. 152–153, 280 (2014).
S. Manchala, V.S.R.K. Tandava, L.R. Nagappagari, S.M. Venkatakrishnan, D. Jampaiah, Y.M. Sabri, S.K. Bhargava, and V. Shanker, Fabrication of a Novel ZnIn2S4/g-C3N4/Graphene Ternary Nanocomposite with Enhanced Charge Separation for Efficient Photocatalytic H2 Evolution Under Solar Light Illumination. Photochem. Photobiol. Sci. 18, 2952 (2019).
C.W. Huang, V.H. Nguyen, S.R. Zhou, S.Y. Hsu, J.X. Tan, and K.C.W. Wu, Metal–Organic Frameworks: Preparation and Applications in Highly Efficient Heterogeneous Photocatalysis. Sustain. Energy Fuels 4, 504 (2020).
Acknowledgments
The authors gratefully acknowledge support from the National Natural Science Foundation of China (NSFC) (No. 52176178 and 51876018), the Innovation Research Group of Universities in Chongqing (No. CXQT21035), and the Action Plan for High-quality Development of Graduate Education of Chongqing University of Technology (No. gzltd202204 and gzlcx20223157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mou, J., Xu, Y., Zhong, D. et al. Efficient Visible-Light-Responsive Photocatalytic Fuel Cell with a ZnIn2S4/UiO-66/TiO2/Ti Photoanode for Simultaneous RhB Degradation and Electricity Generation. J. Electron. Mater. 51, 6121–6133 (2022). https://doi.org/10.1007/s11664-022-09878-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09878-z