Abstract
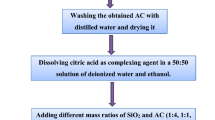
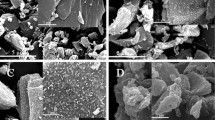
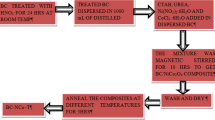
Mesoporous MnO2/activated carbon (AC) nanocomposites are promising materials as anode material in lithium ion batteries which are being considered by researchers due to their low conductivity and considerable irreversible capacity loss. The porous structures of these nanocomposites can facilitate Li ion diffusion into the porous structure. In this paper, MnO2/AC nanocomposites were synthesized by the coating of a MnO2 layer on AC using the reduction reaction of KMnO4 with AC and citric acid. The AC in nanocomposites prepared from walnut shell powder, hydrazine hydrate, and NaCl were used as activating agents. The MnO2/AC nanocomposites were synthesized with mass ratios of 1:4, 1:1, 4:1, and 1:0 and the effect of the annealing process at the temperature of 300°C was investigated. The x-ray diffraction (XRD) patterns of MnO2/AC nanocomposites have shown the growth of the layered birnessite-type MnO2 nanoparticles on the AC. Field emission-scanning electron microscopy (FE-SEM) images of the AC shows cracked surfaces with pieces of sizes from 20 nm to 100 nm and the pore size in the wide range of 20–200 nm. Based on EDS results, decreasing the AC content in MnO2/AC nanocomposites led to the decrease of the weight ratio of carbon before annealing, but increased the weight ratio of carbon after annealing. Fourier-transform infrared (FTIR) spectroscopy results showed the existence of bands attributed to the lattice vibration of Mn−O and the strengthening of the related carbon bands in composites containing AC. The direct and indirect band gaps of MnO2/AC nanocomposites were determined by UV-Vis absorption spectroscopy. For the MnO2/AC nanocomposites with less or equal MnO2 content, the indirect energy gap of MnO2 (≈ 2.4 eV) increases with increasing the MnO2 /AC ratio before annealing, while this gap disappeared after annealing. The direct energy gap of MnO2 in the nanocomposites was always larger than 3.09 eV, due to the nanoscale size of the MnO2 nanoparticles. Comparisons of the direct gaps of 1:4, 1:1, and 4:1 composites before and after annealing imply that the direct gap decreases from 5.88 eV, 5.51 eV, and 6.41 eV before annealing to 5.52 eV, 5.44 eV, and 5.68 eV after annealing, respectively. Electrochemical measurements including voltage capacity and dQ/dV indicate that the MnO2/AC (1:4) nanocomposite anodes demonstrate more than 89% coulombic efficiency and a specific capacity of 1495 mAh/g in 20 mA/g in the first cycle.



















Similar content being viewed by others
References
Y. Liu, K. Shi, and I. Zhitomirsky, Electrochim. Acta 233, 142 (2017).
D.-S. Bin, S.-Y. Duan, X.-J. Lin, L. Liu, Y. Liu, Xu. Yan-Song, Y.-G. Sun, X.-S. Tao, A.-M. Cao, and L.-J. Wan, Nano Energy 60, 912 (2019).
G.A.M. Ali, M.R. Thalji, W.C. Soh, H. Algarni, and K.F. Chong, J. Solid-State Electrochem. 24, 25 (2020).
M.R. Thalji, G.A.M. Ali, P. Liu, Y.L. Zhong, and K.F. Chong, Chem. Eng. J. 409, 128216 (2021).
G.A.M. Ali, M.M. Yusoff, H. Algarni, and K.F. Chong, Ceramic Int. 44, 7799 (2018).
L. Naderi, S. Shahrokhian, and F. Soavi, J. Mater. Chem. A 8, 19588 (2020).
A. Abbasnezhad, H. Asgharzadeh, A. Ansari-Hamedani, and S.H. Soytas, Dalton Trans. 49, 5890 (2020).
Y. Wu, R. Shu, J. Zhang, Z. Wan, J. Shi, Y. Liu, G. Zhao, and M. Zheng, J. Alloys Compd. 819, 152944 (2020).
H. Liu, X. Li, and G. Xing, Int. J. Electrochem. Sci. 15, 12220 (2020).
A. Abdollahi, A. Abnavi, F. Ghasemi, S. Ghasemi, Z. Sanaee, and S. Mohajerzadeh, Electrochim. Acta 390, 138826 (2021).
P.C. Wang, V. Govindan, C.H. Chiang, and C.G. Wu, Solar RPL 4, 2000247 (2020).
A. Ramadan, M. Anas, S. Ebrahim, M. Soliman, and A.I. Abou-Aly, Int. J. Hydrogen Energy 45, 16254 (2020).
N. Wang, W. Li, J. Liang, Y. Huang, Q. Cai, M. Hu, Y. Chen, and Z. Shi, J. Alloys Compd. 846, 156396 (2020).
M. Ates, and O. Kuzgun, Plastics. Rubber Composites 49, 342 (2020).
S. Sekar, S. Lee, P. Vijayarengan, K.M. Kalirajan, T. Santhakumar, S. Sekar, and S. Sadhasivam, Nanomaterials 10, 1610 (2020).
S.M. Hong, E. Jang, A.D. Dysart, V.G. Pol, and K.B. Lee, Sci. Rep. 6, 34590 (2016).
N. Mojoudi, N. Mirghafari, M. Soleimani, H. Shariatmadari, C. Belver, and J. Bedia, Sci. Rep 9, 19352 (2019).
O. Boujibar, F. Ghamouss, A. Ghosh, O. Achak, and T. Chafik, J. Power Sour. 436, 226882 (2019).
M. Karnan, K. Subramani, N. Sudhan, N. Ilayaraja, and M. Sathish, ACS Appl. Mater. Interfaces. 8, 35191 (2016).
M.S. Shafeeyan, W.M.A.W. Daud, A. Houshmand, and A. Shamiri, J. Anal. Appl. Pyrol. 89, 143 (2010).
P. Pietrowski, I. Ludwiczak, and J. Tyczkowski, Mater. Sci. 18, 158 (2012).
J. Rivera-Utrilla, M. Sánchez-Polo, V. Gómez-Serrano, P.M. Álvarez, M.C.M. Alvim-Ferraz, and J.M. Dias, J. Hazard. Mater. 187, 1 (2011).
Z. Heidarinejad, M.H. Dehghani, M. Heidari, G. Javedan, I. Ali, and M. Sillanpää, Environ. Chem. Lett. 18, 393 (2020).
Y. Gao, Q. Yue, B. Gao, and A. Li, Sci. Total Environ. 746, 141094 (2020).
X. Zhang, X. Sun, H. Zhang, D. Zhang, and Y. Ma, Mater. Chem. Phys. 137, 290 (2012).
R. Liu, E. Liu, R. Ding, K. Liu, Y. Teng, Z. Luo, Z. Li, T. Hu, and T. Liu, Ceram. Int. 41, 12734 (2015).
T. Huang, Z. Qiu, D. Wu, and Z. Hu, Int. J. Electrochem. Sci. 10, 6312 (2015).
J. Zhang, J. Sun, T.A. Shifa, D. Wang, X. Wu, and Y. Cui, Chem. Eng. J. 372, 1047 (2019).
W. Dang, C. Dong, Z. Zhang, G. Chen, Y. Wang, and H. Guan, Electrochim. Acta 217, 16 (2016).
L. Li, Q. Lu, J. Xiao, J. Li, H. Mi, R. Duan, J. Li, W. Zhang, X. Li, S. Liu, K. Yang, M. Wu, and Y. Zhang, J. Power Sources 363, 9 (2017).
K. Ahmad, A. Mohammad, and S.M. Mobin, Electrochim. Acta 252, 549 (2017).
K. Gong, P. Yu, L. Su, S. Xiong, and L. Mao, J. Phys. Chem. C 111, 1882 (2007).
S. Orsini, E. Pargoletti, A. Vertova, A. Minguzzi, C. Locatellia, S. Rondinini, and G. Cappelletti, J. Electroanal. Chem. 808, 439 (2018).
E. Pargoletti, V. Pifferi, L. Falciola, G. Facchinetti, A. Re Depaolini, E. Davoli, M. Marelli, and G. Cappelletti, Appl. Surf. Sci. 472, 118 (2019).
M. Qin, H. Zhao, W. Yang, Y. Zhou, and F. Li, R. Soc. Chem. 6, 23905 (2016).
L. Wang, Y. Wu, S. Liu, Y. Zhang, Y. Chen, H. Ma, Z. Zhu, and J. Zhou, BioResearch 14, 7193 (2019).
Y. Liu, S. Zuo, B. Shen, Y. Wang, and H. Xia, Int. J. Electreochem. Sci. 15, 7646 (2020).
Y. Yang, M. Shi, Y.S. Li, and Z.W. Fu, J. Electrochem. Soc. 159, A1917 (2012).
S. Saha, P. Maji, D.A. Pethsangave, A. Roy, A. Ray, S. Some, and S. Das, Electrochim. Acta 317, 199 (2019).
X. Tan, S. Liu, Q. Guo, J. Zhang, S. Liang, M. He, and J. Luo, Int. J. Energy Res. 44, 4556 (2020).
G.T. Xia, C. Li, K. Wang, and L.W. Li, Sci. Adv. Mater. 11, 1079 (2019).
G.R. Li, Z.P. Feng, Y.N. Ou, D. Wu, R. Fu, and Y.X. Tong, Langmuir 26, 2209 (2010).
N. Mohammadi, K. Pourreza, N. Bahrami Adeh, and M. Omidvar, J. Alloy Compd. 883, 160874 (2021).
S. Burhanuddin, A. Yarmo, and B.M. Yamin, AIP Conf. Proc. 1571, 932 (2013).
C.M. Julien, and A. Mauger, Nanomaterials 7, 396 (2017).
A. Hashem, H. Abuzeid, M. Kaus, S. Indris, H. Ehrenberg, A. Mauger, and C.M. Julien, Electrochim. Acta 262, 74 (2018).
Y.P.S. Putri, and A. Awaluddin, Adv. Eng. Res. 190, 75 (2019).
X. Wang, Y. Yang, L. Tao, and M. He, Chem. Geol. 579, 120336 (2021).
W. Yao, H. Zhou, and Y. Lu, J. Power Sour. 241, 359 (2013).
M. Huang, Y. Zhang, F. Li, L. Zhang, R.S. Ruoff, Z. Wen, and Q. Liu, Sci. Rep. 4, 1 (2013).
K.P. Luchty, and J.L. Mendoza-Cortes, J. Phys. Chem. C 119, 22838 (2016).
G.A.M. Ali, M.M. Yusoff, Y.H. Ng, H.N. Lim, and K.F. Chong, Curr. Appl. Phys. 15, 1143 (2015).
F. Li, Y. Xing, M. Huang, K.L. Li, T.T. Yu, Y.X. Zhang, and D. Losic, J. Mater. Chem. A 3, 7855 (2015).
H. Xia, M. Lai, and L. Lu, J. Mater. Chem. 20, 6896 (2010).
S. Lin, K. Li, K. Chen, and D. Xue, Mater. Focus 2, 53 (2013).
J.H. Park, W.Y. Choi, S. Lee, T.S. Kim, and J.W. Lee, Electrochim. Acta 348, 136310 (2020).
A.B. Fuertes, and M. Sevilla, ACS Appl. Mater. Interfaces. 7, 4344 (2015).
M. Ghaly, F.M.S.E. El-Dars, M.M. Hegazy and R.O. Abdel Rahman, Chemical Engineering Journal, 284, 1373, (2016).
K.D. Kwon, K. Refson, and G. Sposito, Geochim. Cosmochim. Acta 73, 4142 (2009).
L. Xing, C. Cui, C. Ma, and X. Xue, Mater. Lett. 65, 2104 (2011).
A. Yu, H.W. Park, A. Davies, D.C. Higgins, Z. Chen, and X. Xiao, J. Phys. Chem. Lett. 2, 1855 (2011).
J. Chen, Y. Wang, X. He, S. Xu, M. Fang, X. Zhao, and Y. Shang, Electrochim. Acta 142, 152 (2014).
H. Kim, N. Venugopal, J. Yoon, and W.S. Yoon, J. Alloy. Compd. 778, 37 (2019).
A.A. Voskanyan, C.K. Ho, and K.Y. Chan, J. Power Sour. 421, 162 (2019).
H. Zeng, B. Xing, C. Zhang, L. Chen, H. Zhao, X. Han, G. Yi, G. Huang, C. Zhang, and Y. Cao, Energy Fuels 34, 2480 (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaeri, M.A., Bagheri Mohagheghi, M.M. Synthesis and Electrochemical Properties of Layered Birnessite MnO2/Activated Carbon Nanocomposite. J. Electron. Mater. 51, 2412–2432 (2022). https://doi.org/10.1007/s11664-022-09499-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09499-6