Abstract
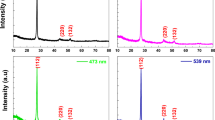
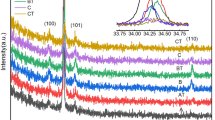
We have fabricated Al-doped ZnO films by a spin-spray method, achieving high conductivity by Al-ion doping and photocatalytic activity of the ZnO. The surface morphology of the as-deposited films was varied by changing the Al concentration and addition of citrate ions. As-deposited Al-doped ZnO film without citrate ions showed rod array structure with increasing rod width as the Al concentration was increased. Meanwhile, Al-doped ZnO film deposited with addition of citrate ions changed to exhibit dense and continuous surface morphology with high transmittance of 85%. The lowest resistivity recorded for undoped and Al-doped ZnO film was 2.1 × 10−2 Ω cm and 5.9 × 10−3 Ω cm, after ultraviolet (UV) irradiation. The reason for the decreased resistivity is thought to be that Al-ion doping and the photocatalytic activity of ZnO contributed to improve the conductivity.
Similar content being viewed by others
References
T. Hirao, M. Furuta, T. Hiramatsu, T. Matsuda, C. Li, H. Furuta, H. Hokari, M. Yoshida, H. Ishii, and M. Kakegawa, IEEE Trans. Electron Devices 55, 3136 (2008).
J.S. Hong, S.M. Kim, S.J. Park, H.W. Choi, and K.H. Kim, Mol. Cryst. Liq. Cryst. 520, 19 (2010).
M. Zhang, F. Jin, M. Zheng, J. Liu, Z. Zhao, and X. Duan, RSC Adv. 21, 10462 (2014).
M.C. Jun, S.U. Park, and J.H. Koh, Nanoscale Res. Lett. 7, 639 (2012).
H. Gomez and M.L. De la Olvera, Mater. Sci. Eng. B 134, 20 (2006).
E. Fortunato, L. Raniero, L. Silva, A. Goncalves, A. Pimentel, P. Barquinha, H. Aquas, L. Pereora, G. Goncalves, I. Ferreira, E. Elangovan, and R. Martins, Sol. Energy Mater. Sol. Cells 92, 1605 (2008).
T. Minami, T. Miyata, Y. Ohtani, and Y. Mochizuki, Jpn. J. Appl. Phys. 45, L409 (2006).
L. Znaidi, Mater. Sci. Eng. B 174, 18 (2010).
S. Baruah and J. Dutta, Sci. Tech. Adv. Mater. 10, 013001 (2009).
J.S. Hong, H. Wagata, N. Ohashi, K. Katsumata, K. Okada, and N. Matsushita, J. Electron. Mater. 44, 2657 (2015).
J.S. Hong, T. Watanabe, H. Wagata, K. Katsumata, K. Okada, and N. Matsushita, J. Jpn. Soc. Powder Powder Metall. 61, S324 (2014).
D.R. Sahu, S. Lin, and J. Huang, Appl. Surf. Sci. 252, 7509 (2006).
J.S. Hong, N. Matsushita, and K.H. Kim, Thin Solid Films 531, 238 (2013).
H. Yaghoubi, Self-Cleaning Materials and Surfaces: A Nanotechnology Approach (London: Wiley, 2013).
J.S. Hong, H. Wagata, K. Katsumata, K. Okada, and N. Matsushita, Jpn. J. Appl. Phys. 52, 110108 (2013).
J.H. Lee, J.W. Lee, S.Y. Hwang, S.Y. Kim, H.K. Cho, J.Y. Lee, and J.S. Park, J. Nanosci. Nanotech. 12, 5598 (2012).
M.H. Mamat, M.Z. Sahdan, Z. Khusaimi, A. Zain Ahmed, S. Abdullah, and M. Rusop, Opt. Mater. 32, 696 (2010).
H. Wagata, N. Ohashi, T. Taniguchi, K. Katsumata, K. Okada, and N. Matsushita, Cryst. Growth Des. 10, 4968 (2010).
H. Wagata, N. Ohashi, K. Katsumata, H. Segawa, Y. Wada, H. Yoshikawa, S. Ueda, K. Okada, and N. Matsushita, J. Mater. Chem. 22, 20706 (2012).
H. Pan, J.B. Yi, L. Shen, R.Q. Wu, J.H. Yang, J.Y. Lin, Y.P. Feng, J. Ding, L.H. Van, and J.H. Yin, Phys. Rev. Lett. 99, 127201 (2007).
N. Ohashi, Y.G. Wang, T. Ishigaki, Y. Wada, H. Taguchi, I. Sakaguchi, T. Ohgaki, Y. Adachi, and H. Haneda, J. Cryst. Growth 306, 316 (2007).
C.G. Vande Walle, Phys. Rev. Lett. 85, 1012 (2000).
J. Tauc, Amorphous and Liquid Semiconductors (New York: Plenum, 1974).
E.A. David and N.F. Mott, Philos. Magn. 22, 903 (1970).
E. Burstein, Phys. Rev. 93, 632 (1954).
K.G. Saw, N.M. Aznan, F.K. Yam, S.S. Ng, and S.Y. Pung, PLoS One 10, e0141180 (2015).
G. Tang, H. Liu, and W. Zhang, Adv. Mater. Sci. Eng. 2013, 348601 (2013).
J.J. Beltran, J.A. Barrero, and A. Punnuoose, Phys. Chem. Chem. Phys. 17, 15284 (2015).
R. Wegmuller, F. Tay, C. Zeder, M. Brnic, and R.F. Hurrell, J. Nutr. 144, 132 (2014).
H. Zhou, D. Yi, Z. Yu, L. Xiao, and J. Li, Thin Solid Films 515, 6909 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, J., Katsumata, Ki. & Matsushita, N. Fabrication of Al-Doped ZnO Film with High Conductivity Induced by Photocatalytic Activity. J. Electron. Mater. 45, 4875–4880 (2016). https://doi.org/10.1007/s11664-016-4751-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-016-4751-7