Abstract
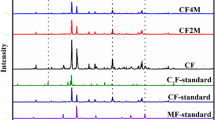
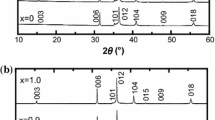
Ca2Fe2−xAlxO5 solid solution is an intermediate product for reduction of complex calcium ferrites, which is a restrictive step for efficient metallurgy of high-aluminum iron ore for ironmaking. In this work, three Ca2Fe2−xAlxO5 (x = 0.00, 0.21, 0.41) solid solutions were synthesized to investigate the reduction mechanism of Ca2Fe2−xAlxO5 solid solution. Combined with the Rietveld refinement method, it was confirmed Ca2Fe2−xAlxO5 is a substitutional solid solution, where the Al3+ could replace the position of Fe3+ to lower the cell size with orthorhombic crystal. The reduction result of Ca2Fe2−xAlxO5 shows that the solid solution of Al3+ in Ca2Fe2−xAlxO5 increased the structure stability, in which the reduction-beginning temperature increased from 820 °C to 1020 °C when x increased to 0.41, which was also verified by first principle calculation. It was found the reduction products of Ca2Fe2−xAlxO5 were iron, CaO, and Ca3Al2O6, where the CaO belongs to a solid solution with the lower melting-temperature, resulting in decrease of the reduction rate at the higher temperature. Furthermore, it was revealed that the Al3+ has been gradually dissolved into Ca2Fe2−xAlxO5 remained with the reduction in progress at the earlier stage, but the Al3+ separates from Ca2Fe2−xAlxO5 and reacts with CaO to generate Ca3Al2O6 at the later stage of reduction due to the Al3+ amount exceeding the solid solution limit (x = 0.63 at 1050 °C).
















Similar content being viewed by others
References
L. Lu, O. Ishiyama, T. Higuchi, M. Matsumura, and Higuchi, K: Iron ore sintering, In Iron Ore Woodhead Publishing, 2022, pp. 489–538
N.A. Webster, M.I. Pownceby, R. Fan, and H.E. Brand: ISIJ Int., 2022, vol. 62, pp. 652–57. https://doi.org/10.2355/isijinternational.ISIJINT-2021-502
H. Han, L. Lu, and S. Hapugoda: Miner. Eng., 2023, vol. 197, 108062https://doi.org/10.1016/j.mineng.2023.108062
L. Tomas da Rocha, S. Cho, S.W. Kim, and S.M. Jung: Metall. Mater. Trans. B, 2022, vol. 53, pp. 3306–21. https://doi.org/10.1007/s11663-022-02612-4
L. Niu, Z. Liu, J. Zhang, D. Lan, S. Li, Z. Li, and Y. Wang: Int. J Min. Met. Mater., 2023, vol. 30, pp. 303–13. https://doi.org/10.1007/s12613-022-2484-6
M. Hayashi, D. Zhou, Y. Iwami, T. Higuchi, T. Watanabe, R. Endo, and M. Susa: ISIJ Int., 2022, vol. 62, pp. 1785–91. https://doi.org/10.2355/isijinternational.ISIJINT-2022-107
T.J. Park, J.S. Choi, and D.J. Min: Steel Res. Int., 2019, vol. 90, p. 1900001. https://doi.org/10.1002/srin.201900001
Y. Takeichi, R. Murao, and M. Kimura: ISIJ Int., 2023, vol. 63, pp. 2017–22. https://doi.org/10.2355/isijinternational.ISIJINT-2023-215
X. Chen, W. Wang, D. Yang, H. Zheng, and B. Wang: ISIJ Int., 2023, vol. 63, pp. 261–70. https://doi.org/10.2355/isijinternational.ISIJINT-2022-348
S. Cho, S.W. Kim, and S.M. Jung: ISIJ Int., 2022, vol. 62, pp. 2587–98. https://doi.org/10.2355/isijinternational.ISIJINT-2022-127
Y.B. Chen, Y. Du, Y.F. Guo, and X.M. Guo: Minerals, 2022, vol. 12, pp. 282–94. https://doi.org/10.3390/min12030282
S.H. Lu, J. Pan, S.W. Li, D.Q. Zhu, Z.Q. Guo, Y. Shi, and B.J. Shi: J. Iron. Steel Res. Int., 2023, vol. 30, pp. 635–49. https://doi.org/10.1007/s42243-022-00860-x
X. Jiang, J. Zhao, L. Wang, H. An, Q. Gao, H. Zheng, and F. Shen: ISIJ Int., 2021, vol. 61, pp. 86–92. https://doi.org/10.2355/isijinternational.ISIJINT-2020-243
N.A. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: Metall. Mater. Trans. B, 2012, vol. 43, pp. 1344–57. https://doi.org/10.1007/s11663-012-9740-5
J. Park, E. Kim, I.K. Suh, and J. Lee: Minerals, 2021, vol. 12, pp. 35–49. https://doi.org/10.3390/min12010035
H. Guo and X.M. Guo: Metall. Mater. Trans. B, 2018, vol. 49, pp. 1974–84. https://doi.org/10.1007/s11663-018-1292-x
S. Nicol, J. Chen, M.I. Pownceby, and N.A. Webster: ISIJ Int., 2018, vol. 58, pp. 2157–72. https://doi.org/10.2355/isijinternational.ISIJINT-2018-203
N.A. Webster, D.P. Odea, B.G. Ellis, and M.I. Pownceby: ISIJ Int., 2017, vol. 57, pp. 41–47. https://doi.org/10.2355/isijinternational.ISIJINT-2016-332
N.A. Webster, M.I. Pownceby, and I.C. Madsen: ISIJ Int., 2013, vol. 53, pp. 1334–40. https://doi.org/10.2355/isijinternational.53.1334
B. Cai, T. Watanabe, C. Kamijo, M. Susa, and M. Hayashi: ISIJ Int., 2018, vol. 58, pp. 642–51. https://doi.org/10.2355/isijinternational.ISIJINT-2017-552
T. Harvey, M.I. Pownceby, J. Chen, N.A. Webster, T.B.T. Nguyen, L. Matthews, D. O’Dea, and T. Honeyands: JOM, 2021, vol. 73, pp. 345–55. https://doi.org/10.1007/s11837-020-04452-6
R. Murao and M. Kimura: ISIJ Int., 2022, vol. 62, pp. 1159–67. https://doi.org/10.2355/isijinternational.ISIJINT-2021-559
F. Liao and X.M. Guo: Mater. Res. Express, 2019, vol. 6, 106501https://doi.org/10.1088/2053-1591/ab3633
T. Maeda and Y. Ono: Tetsu-to-Hagané, 1994, vol. 80, pp. 451–56. https://doi.org/10.2355/tetsutohagane1955.80.6_451
T. Maeda and Y. Ono: Tetsu-to-Hagané, 1989, vol. 75, pp. 416–23. https://doi.org/10.2355/tetsutohagane1955.75.3_416
C. Ding, X. Lv, G. Li, C. Bai, S. Xuan, K. Tang, and Y. Chen: ISIJ Int., 2017, vol. 57, pp. 1181–90. https://doi.org/10.2355/isijinternational.ISIJINT-2017-088
J. Xiao, Y. Song, and Y. Li: Minerals, 2023, vol. 13, pp. 566–80. https://doi.org/10.3390/min13040566
H. Krztoń and J. Stecko: In Iron Ores. 2021, IntechOpen
T. Honeyands, J. Manuel, L. Matthews, D. Odea, D. Pinson, J. Leedham, and M.I. Pownceby: Minerals, 2019, vol. 9, pp. 333–49. https://doi.org/10.3390/min9060333
N. Pailhé, A. Wattiaux, M. Gaudon, and A. Demourgues: J. Solid State Chem., 2008, vol. 181, pp. 1040–47. https://doi.org/10.1016/j.jssc.2008.02.009
S. Ichikawa, D. Fujimura, A. Ohbuchi, and T. Nakamura: ISIJ Int., 2016, vol. 56, pp. 2228–35. https://doi.org/10.2355/isijinternational.ISIJINT-2016-392
I.C. Madsen and N.V. Scarlett: R Soc Chem, 2008, vol. 5, pp. 298–331
V. Kahlenberg, H. Krüger, M. Tribus, and B. Anwander: Miner. Petrol., 2021, vol. 115, pp. 137–47. https://doi.org/10.1007/s00710-020-00730-y
K. Momma and F. Izumi: J. Appl. Crystallogr., 2011, vol. 44, pp. 1272–76. https://doi.org/10.1107/S0021889811038970
G.H. La, J.S. Choi, and D.J. Min: Metals, 2021, vol. 11, pp. 839–51. https://doi.org/10.3390/met11050839
Y. Nakamura, N. Shibayama, A. Hori, T. Matsushita, H. Segawa, and T. Kondo: Inorg. Chem., 2020, vol. 59, pp. 6709–16
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (U22A20175 and No. 52304317) for financial support of this research.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, Y., Guo, XM. Displacement and Migration Behavior of Al3+ in Ca2Fe2−xAlxO5 Solid Solution During Reduction Process. Metall Mater Trans B (2024). https://doi.org/10.1007/s11663-024-03120-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11663-024-03120-3