Abstract
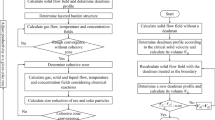
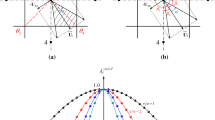
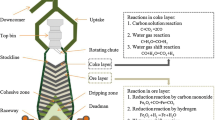
The circumferential uniformity of burden distribution is of critical importance in maintaining the stable and efficient operation of blast furnaces (BFs). It significantly affects the circumferential gas distribution and the reduction process of ferrous materials. However, during the burden-charging process at the furnace top, circumferential non-uniformity inevitably occurs, especially for bell-less top BFs with parallel hoppers. So far, few studies have been reported on the effects of non-uniform burden distribution on the 3D asymmetric inner states of BFs. In this work, the effects of circumferential non-uniform ore-to-coke ratio on the cohesive zone (CZ) shape and location, multiphase flow, thermochemical behaviors, and overall BF performance are numerically studied. This is based on a recently developed 3D computational fluid dynamics (CFD) process model, which features the 3D-layered burden structure and CZ, trickling liquid flow, particle size degradation, and stockline variation. The results show that the high-temperature reducing gas generated in raceways is re-distributed during its ascending process to the furnace top due to the circumferential non-uniform ore-to-coke ratio and, hence, the bed permeability, leading to the increasing temperatures in the low ore-to-coke region but the decreasing ones in the high ore-to-coke region. This asymmetric thermal state results in the 3D inclined CZ, which increases the non-uniformities in gas pressure, and liquid mass flow rate and temperature at the slag surface. In addition, both the sinter reduction degradation and the coke size reduction due to gasification intensify in the high ore-to-coke region but attenuate in the low ore-to-coke region, which increases the BF non-uniformity of bed permeability, and hence, on the multiphase flow and thermochemical behaviors. Moreover, with the increase of circumferential non-uniform degree of ore-to-coke ratio, the average top gas temperature and average tuyere gas pressure slightly increase; however, the average liquid outlet temperature slightly decreases. The model comprehensively illustrates the 3D asymmetric inner states and overall performance resulting from non-uniform burden distribution, which provides an effective tool for BF operation and control in practice.





















Similar content being viewed by others
Change history
14 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11663-023-02831-3
Abbreviations
- \(a_{{\text{FeO}}}\) :
-
The activity of molten wustite
- \(A_{\text{c}}\) :
-
Effective surface area of coke for reaction, \(\text{m}^{2}\)
- \(A_{UD}\) :
-
Circumferential non-uniform degree of ore-to-coke ratio
- \({\text{BF}}\) :
-
Blast furnace
- \(BR\) :
-
Blast rate, \(\text{Nm}^{\text{3}} \cdot {\text{s}}^{ - 1}\)
- \(c_{p}\) :
-
Specific heat, \(\text{J} \cdot \text{kg}^{ - 1} \cdot \text{K}^{ - 1}\)
- \(C_{{\text{SiO}_{2} }}\) :
-
Concentration of \(\text{SiO}_{2}\), \(\text{mol} \cdot \text{m}^{ - 3}\)
- \(CR\) :
-
Coke rate, \(\text{kg} \cdot \text{tHM}^{ - 1}\)
- \({\text{CZ}}\) :
-
Cohesive zone
- \(d\) :
-
Diameter of solid phase, m
- \(D\) :
-
Diffusion coefficient, \(\text{m}^{2} \cdot \text{s}^{ - 1}\)
- \(D_{{\text{s5}}}\) :
-
Intra-particle diffusion coefficient of \({\text{H}}_{{2}}\) in reduced iron phase, \(\text{m}^{2} \cdot \text{s}^{ - 1}\)
- \(E_{\text{f}}\) :
-
Effectiveness factors of solution loss reaction by \({\text{CO}}\)
- \(E_{\text{f}}^{^{\prime}}\) :
-
Effectiveness factors of water gas reaction
- \(E_{{\text{gl}}}\) :
-
Volumetric enthalpy flux between gas and liquid, \(\text{W} \cdot \text{m}^{ - 3}\)
- \(f_{\text{o}}\) :
-
Fraction conversion of iron ore
- \({\mathbf{F}}\) :
-
Interaction force per unit volume, \(\text{kg} \cdot \text{m}^{ - 2} \cdot \text{s}^{ - 2}\)
- \({\mathbf{g}}\) :
-
Gravitational acceleration, \(\text{m} \cdot \text{s}^{ - 2}\)
- \(h_{ij}\) :
-
Heat transfer coefficient between i and j phase, \(\text{W} \cdot \text{m}^{ - 2} \cdot \text{K}^{ - 1}\)
- \(H\) :
-
Enthalpy, \(\text{J} \cdot \text{kg}^{ - 1}\)
- \(\Delta H\) :
-
Reaction heat, \(\text{J} \cdot \text{mol}^{ - 1}\)
- \({\text{HM}}\) :
-
Hot metal
- \(k\) :
-
Thermal conductivity, \(\text{W} \cdot \text{m}^{ - 1} \cdot \text{K}^{ - 1}\)
- \(k_{1}\) :
-
Rate constant of indirect reduction of iron ore by \({\text{CO}}\), \(\text{m} \cdot \text{s}^{ - 1}\)
- \(k_{2}\) :
-
Rate constant of direction reduction of molten wustite, \(\text{mol} \cdot \text{m}^{ - 2} \cdot \text{s}^{ - 1}\)
- \(k_{3}\) :
-
Rate constant of solution loss reaction by \({\text{CO}}\), \(\text{m}^{3} \cdot \text{kg}^{ - 1} \cdot \text{s}^{ - 1}\)
- \(k_{5}\) :
-
Rate constant of indirect reduction of iron ore by \({\text{H}}_{{2}}\), \(\text{m} \cdot \text{s}^{ - 1}\)
- \(k_{6}\) :
-
Rate constant of water gas reaction, \(\text{m}^{3} \cdot \text{kg}^{ - 1} \cdot \text{s}^{ - 1}\)
- \(k_{8}\) :
-
Rate constant of silica reduction reaction in slag, \(\text{m} \cdot \text{s}^{ - 1}\)
- \(k_{{\text{f}}}\) :
-
Gas-film mass transfer coefficient, \(\text{m} \cdot \text{s}^{ - 1}\)
- \(k_{{\text{f5}}}\) :
-
Gas-film mass transfer coefficient in indirect reduction of iron ore by \({\text{H}}_{{2}}\), \(\text{m} \cdot \text{s}^{ - 1}\)
- \(k_{{\text{f6}}}\) :
-
Gas-film mass transfer coefficient water gas reaction, \(\text{m} \cdot \text{s}^{ - 1}\)
- \(K_{1}\) :
-
Equilibrium constant of indirect reduction of iron ore by \({\text{CO}}\)
- \(K_{5}\) :
-
Equilibrium constant of indirect reduction of iron ore by \({\text{H}}_{{2}}\)
- \(LT\) :
-
Liquid temperature, \(\text{K}\)
- \(M_{i}\) :
-
Molar mass of \(i^{{\text{th}}}\) species in gas phase
- \(M_{{\text{sm}}}\) :
-
Molar mass of \({\text{FeO}}\) or flux in solid phase, \({\text{kg}} \cdot {\text{mol}}^{ - 1}\)
- \(N_{{\text{coke}}}\) :
-
Number of coke in unit volume of bed, \(\text{m}^{ - 3}\)
- \(N_{{\text{ore}}}\) :
-
Number of iron oxide in unit volume of bed, \(\text{m}^{ - 3}\)
- \(p\) :
-
Pressure, \(\text{Pa}\)
- \(P\) :
-
Productivity, \(\text{tHM} \cdot \text{m}^{ - 3} \cdot \text{day}^{ - 1}\)
- \(P_{{\text{g}}}\) :
-
Gas pressure, \(\text{kPa}\)
- \(Pe\) :
-
Peclet number
- \(Pr\) :
-
Prandtl number
- \(PR\) :
-
Pulverized coal injection rate, \({\text{kg}} \cdot \text{tHM}^{ - 1}\)
- \(R\) :
-
Gas constant, \(8.314 \text{J} \cdot \text{mol}^{ - 1} \cdot \text{K}^{ - 1}\)
- \(R_{k}^{*}\) :
-
Reaction rate for \(k^{{\text{th}}}\) reaction, \(\text{mol} \cdot \text{m}^{ - 3} \cdot \text{s}^{ - 1}\)
- \(R_{O/(O + C)}\) :
-
Ore-to-coke ratio
- \(RD\) :
-
Reduction degree
- \(RDI\) :
-
Reduction degradation index of sinter, pct
- \(Re\) :
-
Reynolds number
- \(S\) :
-
Source term
- \(Sc\) :
-
Schmidt number
- \(Sh_{r}^{ * }\) :
-
Normalized shrinkage ratio
- \(t_{{\text{s}}}\) :
-
Timeline, \(\text{s}\)
- \(t_{batch}\) :
-
Total batch time for one ore layer and one coke layer, s
- \(T\) :
-
Temperature, \(\text{K}\)
- \(TGP\) :
-
Tuyere gas pressure, \({\text{kPa}}\)
- \(TGT\) :
-
Top gas temperature, \(\text{K}\)
- \(TGUF\) :
-
Top gas utilization factor, pct
- Velocity,\({\mathbf{u}}\) :
-
\(\text{m} \cdot \text{s}^{ - 1}\)
- \(U_{{\text{g}}}\) :
-
Gas velocity, \(\text{m} \cdot \text{s}^{ - 1}\)
- \(V_{O}\) :
-
Bulk volume of ore particles, \(\text{m}^{3}\)
- \(V_{C}\) :
-
Bulk volume of coke particles, \(\text{m}^{3}\)
- \(y_{i}\) :
-
Mole fraction of \(i^{{\text{th}}}\) species in gas phase
- \(y_{{\text{CO}}} ,y_{{\text{H}_{\text{2}} }}\) :
-
Molar fraction of \({\text{CO}}\) and \({\text{H}}_{{2}}\)
- \(y_{{\text{CO}}}^{*} ,y_{{\text{H}_{\text{2}} }}^{*}\) :
-
Molar fraction of \({\text{CO}}\) and \({\text{H}}_{{2}}\) in equilibrium state for indirect reaction
- \(y_{{\text{CO}_{\text{2}} }} ,y_{{\text{H}_{\text{2}} \text{O}}}\) :
-
Molar fraction of \({\text{CO}}_{{2}}\) and \({\text{H}}_{{2}} {\text{O(g)}}\)
- \(\alpha\) :
-
Specific surface area, \(\text{m}^{2} \cdot \text{m}^{ - 3}\);
- \(\Gamma\) :
-
Diffusion coefficient
- \(\varepsilon\) :
-
Volume fraction
- \(\eta\) :
-
Fractional acquisition of reaction heat
- \(\theta\) :
-
Circumferential azimuth degree, \(^\circ\)
- \({\mathbf{I}}\) :
-
Identity tensor
- \(\mu\) :
-
Viscosity, \(\text{kg} \cdot \text{m}^{ - 1} \cdot \text{s}^{ - 1}\)
- \(\xi_{{{\text{ore}}}} ,\xi_{{{\text{coke}}}}\) :
-
Local ore, coke volume fraction
- \(\rho\) :
-
Density, \(\text{kg} \cdot \text{m}^{ - 3}\)
- \(\rho_{bulk}\) :
-
Bulk density of burden at BF throat, \(\text{kg} \cdot \text{m}^{ - 3}\)
- \(\sigma\) :
-
The circumferential non-uniformity of a general variable \(\varphi\)
- \({\varvec{\tau}}\) :
-
Stress tensor, Pa
- \(\varphi\) :
-
General variable
- \(\omega\) :
-
Mass fraction
- \(\text{e}\) :
-
Effective
- \(\text{g}\) :
-
Gas
- i :
-
Sector number
- \(i\text{,}m\) :
-
\(m^{{\text{th}}}\) Species in i phase
- \(j\) :
-
Identifier (g, s or l)
- \(k\) :
-
\(k^{{\text{th}}}\) Reaction
- \(\text{l}\) :
-
Liquid
- \(\text{l,d}\) :
-
Dynamic liquid
- \(\text{s}\) :
-
Solid
- \(\text{sm}\) :
-
FeO or flux in solid phase
- e:
-
Effective
- g:
-
Gas
- l:
-
Liquid
- s:
-
Solid
- T:
-
Transpose
References
M. Geerdes, R. Chaigneau, and O. Lingiardi: Modern Blast Furnace Ironmaking: An Introduction, Ios Press, Amsterdam, 2020.
Y. Omori: Blast Furnace Phenomena and Modelling, Elsevier Science Pub. Co., Inc., New York, 1987.
J. Chen, H. Zuo, Q. Xue, and J. Wang: Powder Technol., 2021, vol. 398, p. 117055.
Y. Xu, J. Xu, C. Sun, K. Ma, C. Shan, L. Wen, S. Zhang, and C. Bai: Powder Technol., 2018, vol. 328, pp. 245–55.
H. Mio, S. Komatsuki, M. Akashi, A. Shimosaka, Y. Shirakawa, J. Hidaka, M. Kadowaki, S. Matsuzaki, and K. Kunitomo: ISIJ Int., 2009, vol. 49, pp. 479–86.
Z.Y. Zhou, H.P. Zhu, B. Wright, A.B. Yu, and P. Zulli: Powder Technol., 2011, vol. 208, pp. 72–85.
M. Kou, S. Wu, H. Zhou, Y. Yu, and J. Xu: ISIJ Int., 2018, vol. 58, pp. 2018–24.
Z.Y. Li, S.B. Kuang, S.D. Liu, J.Q. Gan, A.B. Yu, Y.T. Li, and X.M. Mao: Powder Technol., 2019, vol. 353, pp. 385–97.
Y. Niwa, T. Sumigama, A. Maki, H. Ito, H. Inoue, and T. Tamura: ISIJ Int., 1991, vol. 31, pp. 487–93.
H. Takahashi, H. Kawai, M. Kobayashi, and T. Fukui: ISIJ Int., 2005, vol. 45, pp. 1386–95.
M. Chu, H. Nogami, and J.-I. Yagi: ISIJ Int., 2004, vol. 44, pp. 510–17.
H. Tanaka and T. Harada: Tetsu-to-Hagané, 2006, vol. 92, pp. 1022–28.
Y. Ujisawa, K. Sunahara, Y. Matsukura, K. Nakano, and T. Yamamoto: Tetsu-to-Hagané, 2006, vol. 92, pp. 591–600.
M. Chu, Z. Liu, Z. Wang, and J.I. Yagi: Steel Res. Int., 2011, vol. 82, pp. 521–28.
E.A. Mousa, A. Babich, and D. Senk: ISIJ Int., 2011, vol. 51, pp. 350–58.
X.B. Yu and Y.S. Shen: Energy Fuel, 2019, vol. 33, pp. 11603–16.
Y. Liu, Iron Steel, 1993, vol. 4, pp. 5-10.
X. Huang, Q. Zheng, A. Yu, and W. Yan: Powder Technol., 2021, vol. 377, pp. 350–60.
J. Qiu, D. Ju, J. Zhang, and Y. Xu: Powder Technol., 2017, vol. 314, pp. 218–31.
M. Nomura, S. Taguchi, and M. Kondoh: J. Iron Steel I. Jpn., 1982, vol. 68, p. 38.
M. Nomura, S. Taguchi, N. Tsuchiya, H. Sakimura and K. Tanaka, In Ironm. Conf. Proc., (1984), pp 111-17.
J. Xu, S.L. Wu, M.Y. Kou, L.H. Zhang, and X.B. Yu: Appl. Math. Model., 2011, vol. 35, pp. 1439–55.
G.L. Zhao, S.S. Cheng, W.X. Xu, and C. Li: ISIJ Int., 2015, vol. 55, pp. 2566–75.
H.T. Zhao, M.H. Zhu, P. Du, S. Taguchi, and H.C. Wei: ISIJ Int., 2012, vol. 52, pp. 2177–85.
T.Z. Ren, X. Jin, H.Y. Ben, and C.Z. Yu: J. Iron Steel Res. Int., 2006, vol. 13, pp. 14–17.
S. Watakabe, K. Miyagawa, S. Matsuzaki, T. Inada, Y. Tomita, K. Saito, M. Osame, P. Sikström, L.S. Ökvist, and J.-O. Wikstrom: ISIJ Int., 2013, vol. 53, pp. 2065–71.
J. Yagi: ISIJ Int., 1993, vol. 33, pp. 619–39.
X.F. Dong, A.B. Yu, J. Yagi, and P. Zulli: ISIJ Int., 2007, vol. 47, pp. 1553–70.
S. Ueda, S. Natsui, H. Nogami, J.-I. Yagi, and T. Ariyama: ISIJ Int., 2010, vol. 50, pp. 914–23.
T. Ariyama, S. Natsui, T. Kon, S. Ueda, S. Kikuchi, and H. Nogami: ISIJ Int., 2014, vol. 54, pp. 1457–71.
S. Ghosh, N.N. Viswanathan, and N.B. Ballal: Steel Res. Int., 2017, vol. 88, p. 1600440.
S.B. Kuang, Z.Y. Li, and A.B. Yu: Steel Res. Int., 2018, vol. 89, p. 1700071.
B. Xu, A. Yu, S. Chew, and P. Zulli: Powder Technol., 2000, vol. 109, pp. 13–26.
H. Nogami, H. Yamaoka, and K. Takatani: ISIJ Int., 2004, vol. 44, pp. 2150–58.
S. Sarkar, G.S. Gupta, and S.-Y. Kitamura: ISIJ Int., 2007, vol. 47, pp. 1738–44.
Y. Shen, B.-Y. Guo, A. Yu, P.R. Austin, and P. Zulli: Fuel, 2011, vol. 90, pp. 728–38.
B. Guo, D. Maldonado, P. Zulli, and A. Yu: ISIJ Int., 2008, vol. 48, pp. 1676–85.
L. Shao and H. Saxen: Steel Res. Int., 2012, vol. 83, pp. 197–204.
L. Shao and H. Saxen: Ind. Eng. Chem. Res., 2013, vol. 52, pp. 5479–488.
L. Shao and H. Saxen: ISIJ Int., 2013, vol. 53, pp. 1756–62.
L. Shao and H. Saxen: ISIJ Int., 2013, vol. 53, pp. 988–94.
H. Mio, M. Kadowaki, S. Matsuzaki, and K. Kunitomo: Miner. Eng., 2012, vol. 33, pp. 27–33.
T. Mitra and H. Saxén, ISIJ Int., 2016, pp. ISIJINT-2016-114.
S.D. Liu, Z.Y. Zhou, K.J. Dong, A.B. Yu, D. Pinson, and J. Tsalapatis: Steel Res. Int., 2015, vol. 86, pp. 651–61.
P.R. Austin, H. Nogami, and J. Yagi: ISIJ Int., 1997, vol. 37, pp. 458–67.
P.R. Austin, H. Nogami, and J. Yagi: ISIJ Int., 1997, vol. 37, pp. 748–55.
K. Takatani, T. Inada, and Y. Ujisawa: ISIJ Int., 1999, vol. 39, pp. 15–22.
J.A. de Castro, H. Nogami, and J. Yagi: ISIJ Int., 2002, vol. 42, pp. 44–52.
X.F. Dong, A.B. Yu, S.J. Chew, and P. Zulli: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 330–49.
K. Yang, S. Choi, J. Chung, and J. Yagi: ISIJ Int., 2010, vol. 50, pp. 972–80.
J.A. de Castro, A.J. da Silva, Y. Sasaki, and J. Yagi: ISIJ Int., 2011, vol. 51, pp. 748–58.
D. Fu, Y. Chen, Y.F. Zhao, J. D’Alessio, K.J. Ferron, and C.Q. Zhou: Appl. Therm. Eng., 2014, vol. 66, pp. 298–308.
S.B. Kuang, Z.Y. Li, D.L. Yan, Y.H. Qi, and A.B. Yu: Miner. Eng., 2014, vol. 63, pp. 45–56.
Y.S. Shen, B.Y. Guo, S. Chew, P. Austin, and A.B. Yu: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 432–48.
P. Zhou, H.L. Li, P.Y. Shi, and C.Q. Zhou: Appl. Therm. Eng., 2016, vol. 95, pp. 296–302.
L.L. Jiao: Chemical Engineering, Monash University, Melbourne, 2020.
L.L. Jiao, S.B. Kuang, A.B. Yu, Y.T. Li, X.M. Mao, and H. Xu: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 258–75.
S.J. Zhang, A.B. Yu, P. Zulli, B. Wright, and U. Tüzün: ISIJ Int., 1998, vol. 38, pp. 1311–19.
Z.Y. Zhou, A.B. Yu, and P. Zulli: Prog. Cmput. Fluid Dyn., 2004, vol. 4, pp. 39–45.
L.L. Jiao, S.B. Kuang, L.L. Liu, A.B. Yu, Y.T. Li, X.M. Mao, and H. Xu: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 138–55.
T. Kon, S. Natsui, S. Ueda, and H. Nogami: ISIJ Int., 2015, vol. 55, pp. 1284–90.
S. Natsui, A. Sawada, K. Terui, Y. Kashihara, T. Kikuchi, and R.O. Suzuki: Chem. Eng. Sci., 2018, vol. 175, pp. 25–39.
S. Ergun: Ind. Eng. Chem., 1953, vol. 45, pp. 477–85.
G.X. Wang, S.J. Chew, A.B. Yu, and P. Zulli: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 333–43.
W. Ranz and W. Marshall: Prog, 1952, vol. 48, p. 173.
T. Akiyama, R. Takahashi, and J. Yagi: ISIJ Int., 1993, vol. 33, pp. 703–10.
P. Mackey and N. Warner: Metall. Mater. Trans. B, 1972, vol. 3B, pp. 1807–16.
C. Rhie and W.L. Chow: AIAA J., 1983, vol. 21, pp. 1525–32.
S. Patankar: Numerical Heat Transfer and Fluid Flow, Taylor & Francis, New York, 2018.
G. Usera, A. Vernet, and J. Ferré: Flow Turbul. Combust., 2008, vol. 81, p. 471.
L.L. Jiao, S.B. Kuang, Y.T. Li, X.M. Mao, H. Xu, and A.B. Yu: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 2642–58.
I. Muchi: T. Iron Steel I. Jpn., 1967, vol. 7, pp. 223–37.
Acknowledgments
The authors are grateful to the Baosteel Australia Research and Development Center (BAJC) (BA16002), the National Natural Science Foundation of China (52034003), and Natural Science Foundation of Jiangsu Province (BK20210007) for the financial support of this work, and the National Computational Infrastructure (NCI) for the use of high-performance computational facilities, and CAFFA3D for making a useful code available for free use and adaptation.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiao, L., Kuang, S., Li, Y. et al. Numerical Simulation of the 3D Asymmetric Inner States of an Ironmaking Blast Furnace Resulting From Circumferential Non-uniform Burden Distribution. Metall Mater Trans B 54, 734–755 (2023). https://doi.org/10.1007/s11663-023-02722-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02722-7