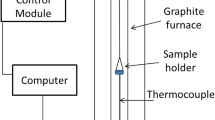
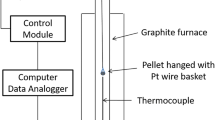
Abstract
Oxidation of magnetite pellets is commonly performed to prepare strong pellets for ironmaking. This article presents a contribution to quantitative understanding of fundamental pellet oxidation kinetics, based on measured oxidation kinetics of magnetite particles and pellets. The commonly observed “plateau” oxidation behavior is confirmed to be consistent with the effect of very large differences in magnetite particle sizes in the concentrate from which pellets are produced. The magnetite particles range in size from less than a micron to several tens of a microns; changing the size distribution by inert sintering of pellets decreases both the plateau level of oxidation and the specific surface area, in ways that are compatible with an assumed Rosin-Rammler magnetite particle size distribution.








Similar content being viewed by others
References
H.J. Cho and P.C. Pistorius: AISTech 2011 Proceedings Volume I, Association for Iron and Steel Technology, Warrendale, PA, 2011, pp. 507-14.
S.R.B. Cooke and T.E. Ban: Mining Eng., 1952, vol. 6, pp. 1052-58.
H. Kokal: MSc Thesis, University of Minnesota, Minneapolis, 1970.
J.D. Zetterstrom: Bureau of Mines Report of Investigations 4728, United States Department of the Interior, Washington, DC, 1950.
M. Tang, H.J. Cho, and P.C. Pistorius: Metall. Mater. Trans. B, in press.
S.P.E. Forsmo, S.-E. Forsmo, P.-O. Samskog and B.M.T. Björkman: Powder Technol., 2008, vol. 183, pp. 247-59.
B.E. Monsen, S.E. Olsen and L. Kolbeinsen: Scand. J. Metall., 1994, vol. 23, pp. 74-80.
V. Niiniskorpi: Academic dissertation, Åbo Akademi, Åbo, Laboratory of Inorganic Chemistry, 2004, pp 54–55.
D. Papanastassiou and G. Bitsianes: Metall. Trans., 1973, vol. 4, pp. 487-96.
K. Meyer, H. Rausch, and M. Ottow: Stahl und Eisen, 1967, vol. 87, pp. 654-60.
P. Rosin and E. Rammler: J. Inst. Fuel, 1933, vol. 7, pp. 29-36.
K.M. Djamarani and I.M. Clark: Powder Technol., vol. 93, 1997, pp. 101-8.
H.P. Klug and L.E. Alexander: X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed., Wiley, New York, NY, 1974, pp.493-94.
H.J. Cho and P.C. Pistorius: AISTech 2012 Proceedings, Association for Iron and Steel Technology, Warrendale, PA, 2012, pp. 503-11.
J. Païdassi: Acta Metall., 1958, vol. 6, pp. 219-21.
G.J. Yurek, J.P. Hirth, and R.A. Rapp: Oxid. Met., 1974, vol. 8, pp. 265-81.
Acknowledgments
Support of this project by the members of the Center for Iron and Steelmaking Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 13, 2013.
Rights and permissions
About this article
Cite this article
Cho, H.J., Tang, M. & Pistorius, P.C. Magnetite Particle Size Distribution and Pellet Oxidation. Metall Mater Trans B 45, 1213–1220 (2014). https://doi.org/10.1007/s11663-014-0104-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0104-1