Abstract
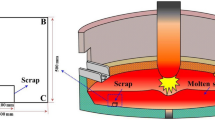
The scrap dissolution in an actual process like the BOF is affected both by mass transfer and heat transfer. In this paper, the mass transfer of carbon in liquid melt is considered along with heat transfer. The approaches used in this paper to model the scrap dissolution phenomenon include the application of Green’s function, quasi-static, integral profile, and the finite difference approach for different Biot numbers. Mass transfer coefficients are calculated using the Chilton–Colburn’s analogy for the case of forced convection. Since the quasi-static approach requires the least computational time, it is used for a detailed parametric study, including the effect of other parameters like different scrap ratios and heating rates of liquid melt. The region of control of heat transfer vs mass transfer is also identified. The dissolution of mixed scrap (light and heavy scrap) is investigated for different scrap ratios and the autogenous heating rates of liquid melt, with the help of mathematical models. The heat transfer coefficient is estimated as a function of mixing energy and the mass transfer coefficient by invoking the Chilton–Colburn analogy. The permissible limits of light scrap, which can be charged into the BOF, are also suggested from the results of this model. The Artificial Neural Network (ANN) model is trained on the dataset (patterns) generated by the coupled heat and mass transfer model. The accuracy of the results obtained using different ANN topologies is discussed followed by a recommendation for selecting the best approach.



























Similar content being viewed by others
Abbreviations
- \(\rho\) :
-
Mass density of the scrap (Kg/m3)
- \( \alpha \) :
-
Thermal diffusivity of solid scrap (m2/s)
- h :
-
Heat transfer coefficient between liquid and solid (W/m2/K)
- β :
-
Mass transfer coefficient between liquid and solid (m/s)
- ν:
-
The moving velocity of the interface (solidification/melting rate) (m/s)
- ΔH :
-
The latent heat of melting (J/Kg)
- k :
-
Thermal conductivity in the solid steel (W/m/K)
- L :
-
Half length of scrap (m)
- T m :
-
The interface temperature of scrap and liquid metal (K)
- T s :
-
Inside temperature of scrap (K)
- T b :
-
Temperature of bulk liquid metal (K)
- C b :
-
Carbon content of bulk liquid metal (pct)
- C i :
-
Carbon content of liquid metal at the scrap–liquid metal interface (pct)
- C s :
-
Carbon content inside the scrap at the scrap–liquid metal interface (pct)
- Bi :
-
Biot number
- C sc :
-
Carbon content of the parent scrap (pct)
- Ph :
-
Phase transformation number
- \( \theta_{{T_{\text{b}} }} \) :
-
Nondimensional bulk liquid temperature
- p :
-
Nondimensional time
- \( \theta \) :
-
Nondimensional temperature
- x′:
-
Nondimensional location
- G′:
-
Green’s function
- Fo_fdm:
-
Fourier number defined for the FDM approach
- t m :
-
Total dissolution time (s)
- \( t^{\prime}_{\text{m}} \) :
-
Nondimensional total dissolution time
- \( \gamma \) :
-
The distribution ratio of carbon between the solid and liquid as given by Fe-C diagram
- D C :
-
The diffusion coefficient of carbon in liquid melt (m2/s)
- C pm :
-
Specific heat of liquid metal (J/Kg/K)
- W m :
-
Weight of liquid metal (Kg)
- t:
-
Time (s)
- h o :
-
Heat transfer coefficient for non-moving boundary, W/m2/K
- Sh :
-
Sherwood number
- Re :
-
Reynold’s number
- Sc :
-
Schmidt number
- Pr :
-
Prandtl number
- Nu :
-
Nusselt number
References
S. Asai and I. Muchi: Trans. ISIJ, 1971, vol. 11, pp 107–15.
H.W. Hartog, P.J. Kreyger and A.B. Snoeijer (1973) CRM 37:13–21.
H. Gaye, J. Wanin, P. Gugliermina, and P Schittly: Proceedings of the 68th Steelmaking Conference, Detroit, April 14–17, 1985, pp. 91–102.
L.C. Brabie and M. Kawakami, High Temp. Mater. Processes (London), 2000, vol. 19 (3/4), pp 531–38.
K. Mori and H. Nomura: Tetsu-to-Hagane, 1969, vol. 55, (5), pp 347–54.
M. Kawakami, K. Takatani and L.C. Brabie (1999) Tetsu-to-Hagane 85(9):658–65.
K. Isobe, H. Maede, K. Ozawa, K. Umezawa and C. Saito (1990) Tetsu-to-Hagane 76(11):2033–40.
M. Kosaka and S. Minova (1967) Tetsu-to-Hagane 53 (8):983–97.
Y.U. Kim and R.D. Pehlke: Metallurgical Transactions B, 1975, vol. 6B, pp 585–91.
H. Yorucu and R. Rolls: Iron Steel Int., 1976, vol 2. pp 35–40.
L. Zhang and F. Oeters (1999) Melting and mixing of alloying agents in steel melts, Verlag Stahleisen Gmbh, Dusseldorf, pp. 87–91.
L. Zhang and F. Oeters (2000) Steel Res 71 (5):141–44.
J.K. Wright: Metall. Mater. Trans. B, 1989, vol. 20B, pp 363–74.
B. Deo, G. Gupta, and M. Gupta: Proceedings of the Asia Steel International Conference, Jamshedpur India, April 9–12, vol. 02, 2003, pp. 2.d.1.1–2.d.1.8.
G.K. Gupta: B. Tech Report, Department of Materials and Metallurgical Engineering, IIT Kanpur, 1998.
E. Specht and R. Jeschar, Steel Res., vol. 64, 1993, pp. 28–34.
R.I.L. Guthrie and P. Stubbs: Can. Metall. Q., 1973, vol. 12 (4), pp 465–73.
R.I.L. Guthrie and L. Gourtsoyannis: Can. Metall. Q., 1972, vol. 10 (1), pp 37–46.
A.K. Shukla: Ph.D. thesis, IIT Kanpur, 2011.
Y. Wanibe, S. Takai, T. Fujisawa and H. Sakao: Trans. ISIJ, 1982, vol. 22, pp 560–65.
A.K. Shukla, B.Deo, S. Millman, B. Snoeijer, A. Overbosch and A. Kapilasharami: Steel Res. Int., 2010, vol. 81(11), pp 940–48.
T. Oya: Tetsu-to-Hagane, 1965, vol. 51, pp 1925.
R. Hill: M.S. Thesis, University of Illinois at Chicago Circle, 1974.
V.P. Bondarenko, A.M. Pozhivanov, V.F. Mikhnevich and V.V. Smoktii: Steel USSR, 1973, vol. 11, pp 900–05.
K. Nakanishi, T. Fujii, and J. Szekely: Ironmaking and Steelmaking, 1975, vol.. 3, pp. 193–97.
K. Chihara and S. Murakami: Proceedings of the Combined Blowing Processes in Nippon Steel Conference, France, 1984, pp 2.4.1–2.4.17.
N. Rajesh, M. R. Khare and S.K. Pabi: Mater. Res. 2010, vol. 13(1), pp 15–19.
A.M.F. Fileti, T.A. Pacianotto and A. P. Cunha: Engineering Applications of Artificial Intelligence, 2006, vol. 19, pp 9–17.
A. Das, J. Maiti, R.N. Banerjee: Expert Systems with Applications, 2010, vol. 37, pp 1075–85.
I.J. Cox, R.W. Lewis, R.S. Ransing, H. Laszczewski, G. Berni: Journal of Materials Processing Technology, 2002, vol. 120, pp 310–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 12, 2011.
Appendices
Appendix A: Estimation of Heat and Mass Transfer Coefficients as a Function of Velocity of the Moving Interface

Stagnant concentration and thermal boundary layers in liquid melt are assumed to exist adjacent to the interface. The heat transfer in the boundary layer takes place only through conduction, whereas the mass transfer occurs through diffusion.
Governing Mathematical Equations for Mass Transfer
It is assumed that the mass transfer in the boundary layer occurs only due to the diffusion process. The mass transfer equation in 1-D in the liquid melt adjacent to the scrap interface is given by
with the following boundary conditions:
where the thickness of the concentration boundary layer \( \delta_{\text{c}} \) is estimated from
which means that the thickness of the boundary layer is given by
where D is the diffusion coefficient for mass transfer in the liquid melt and \( \beta_{0} \) is the mass transfer coefficient.
The solution of Eq. [A1] is
The mass transfer of carbon from the melt to the interface per unit time unit area is calculated from
\( {\text{Mass}}\;{\text{flux}}\;{\text{of}}\;{\text{carbon}}\;\left( {{\text{unit}}\;{\text{time}}\;{\text{unit}}\;{\text{area}}} \right) = \left. {D\frac{dC}{dX}} \right|_{X = 0} = D(C_{\text{b}} - C_{\text{i}} )\left( \frac{v}{D} \right)\left. {\frac{\exp ( - vX/D)}{{1 - \exp ( - v\delta_{\text{C}} /D)}}} \right|_{X = 0} = (C_{\text{b}} - C_{\text{i}} )\frac{v}{1 - \exp ( - v\delta /D)}. \)
Substituting the value of \( \delta_{\text{C}} \),
the mass flux of carbon can be calculated from
Equation [A4] can be rewritten as
where β is the mass transfer coefficient for the case of the moving boundary.
From Eqs. [A4] and [A5], the mass transfer coefficient for the moving boundary is estimated from
Governing Equation for Heat Transfer
It is assumed that the heat transfer in the boundary layer occurs due to conduction. The heat transfer equation in 1-D in the liquid melt adjacent to the scrap interface is given by
where
with following conditions:
where \( \delta_{T} \) is the thickness of the thermal boundary layer which is estimated from the following equation:
It implies that the thickness of the boundary layer is given by
where k is the thermal conductivity in the liquid melt and \( h_{\text{o}} \) is the heat transfer coefficient.
The solution of Eq. [A7] is
The heat transfer flux at the interface (J H) is given by
Putting the value of \( \delta_{T} \) and \( \alpha \),
Equation [A10] may be rewritten as
where h is the heat transfer coefficient for the moving boundary.
From Eqs. [A8], [A10], and [A11], the heat transfer coefficient for the moving boundary can be estimated from
Heat and Mass Balance at the Interface
Heat balance at the interface is given as
On substituting the value of h from Eq. [A12], the above equation can be written as
Mass balance of carbon at the interface is given by
On substituting the value of β from Eq. [A6], the above equation becomes
Equations [A14] and [A16] are coupled together as
Appendix B
Estimation of Heat Transfer Coefficient as a Function of Total Mixing Energy to the BOF Steelmaking System[25,26]
The following equations are used to estimate heat transfer coefficient as a function of total mixing energy to the BOF steelmaking system which is considered under combined influence of top lance, bottom stirring flow rate, and decarburization reaction.
where \( \phi \) is the angle of the lance tip from vertical, \( Q_{\text{t}} \) is the oxygen flow rate, W is the weight of steel, n is the number of openings of the lance, X is the lance height above the metal bath during blowing, H is the bath depth, h_frac is the average depth fraction at which CO bubble formation takes place, \( d_{\text{t}} \) is the throat diameter, \( T_{\text{o}} \) is the temperature of the bottom stirring gas at input, \( T_{\text{l}} \) is the average temperature of the liquid steel, \( p_{\text{atm}} \) is the atmospheric pressure, and h is the heat transfer coefficient.
Process Conditions Used in Simulation
Lance angle = 14 deg Number of openings in lance = 6 Throat diameter = 2.46 cm Bath depth = 1.30 m | bottom stirring flow rate = 2 Nm3/min (first 30 pct and last 30 pct of the blow) blowing pattern is defined as follows: oxygen flow rate = 500 NM3/min if 0 < t < 180 seconds; Lance height = 2.20 m if 180 < t < 300 seconds; Lance height = 2.00 m if 300 < t < 400 seconds; Lance height = 1.80 m if t > 400 seconds; Lance height = 1.60 |
Appendix C: Artificial Neural Network
The concept of ANN originally comes from the mechanisms for information processing in the human brain system. ANN models have been applied to a wide range of complex metallurgical processes[27–30] and proved to be successful due to their ability to develop nonlinear relationships. ANNs are the mathematical patterns constructed by several neurons arranged in different layers interconnected through the complex networks. The layers are defined as input layer, output layer, and at least one hidden layer. A multilayer feed forward backpropagation ANN network has been used in the present work. The typical ANN topology is presented in Figure A1.
The output of a neuron (k) in the network (y k ) is the summation of all signals from a previous layer multiplied by weights (w k,j ) and a bias (b k ) which is activated by a transfer function (tanh sigmoid) in the following way:
The sum of the square of the errors (between the training output data and output data obtained using ANN) is minimized for getting the correct values of weights.
Rights and permissions
About this article
Cite this article
Shukla, A.K., Deo, B. & Robertson, D.G.C. Scrap Dissolution in Molten Iron Containing Carbon for the Case of Coupled Heat and Mass Transfer Control. Metall Mater Trans B 44, 1407–1427 (2013). https://doi.org/10.1007/s11663-013-9905-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9905-x