Abstract
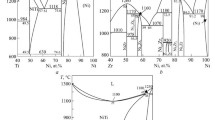
The isothermal section of the Mn-Sn-Zn system at 500 °C was determined with 20 alloys. The alloys were prepared by melting the pure elements in evacuated quartz capsules. The alloy samples were examined by means of X-ray diffraction (XRD) and scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy. A new ternary phase Mn4Zn8Sn (λ) was found to have a bcc structure with a lattice parameter a = 0.92508 (5) nm. Its composition range spans 25 to 35 at. pct Mn, 4 to 8 at. pct Sn, and 55 to 70 at. pct Zn. The Zn is substituted for Mn in Mn3Sn, Mn2Sn, and Mn3Sn2. The solubility of Zn in Mn3Sn, Mn2Sn, and Mn3Sn2 was measured to be about 17, 12, and 4 at. pct, respectively. The phase boundaries of the liquid and β-Mn phases were well established. The following 3 three-phase equilibria were well determined: (1) β-Mn + ε-MnZn3 + Mn3Sn, (2) λ + Mn3Sn + Mn2Sn, and (3) L + λ + Mn2Sn. The additional 5 three-phase equilibria, which are ε-MnZn3 + λ + Mn3Sn, ε 1-MnZn3 + ε-MnZn3 + λ, ε 1-MnZn3 + λ + L, Mn2Sn + L + MnSn2, and Mn3Sn2 + MnSn2 + Mn2Sn, were deduced and shown with dashed lines in the present isothermal section.















Similar content being viewed by others
References
N.Y. Tang: J. Phase Equilib., 2008, vol. 29, pp. 337–44.
G. Reumont, P. Perrot, and J. Foct: J. Mater. Sci., 1998, vol. 33, pp. 4759–68.
N. Pistofidis, G. Vourlias, S. Konidaris, E. Pavlidou, A. Stergiou, and G. Stergioudis: Mater. Lett., 2006, vol. 60, pp. 786–89.
N. Pistofidis, G. Vourlias, E. Pavlidou, K. Chrissafis, G. Stergioudis, E.K. Polychroniadis, and D. Tsipa: J. Therm. Anal. Calorim., 2006, vol. 86, pp. 417–22.
G. Reumont, T. Gloriant, and P. Perrot: J. Mater. Sci. Lett., 1996, vol. 15, pp. 445–49.
R.W. Sandelin: Wire Wire Products, 1940, vols. 15–11, pp. 655–76.
N.Y. Tang: J. Phase Equilib., 2006, vol. 27, pp. 462–68.
N.Y. Tang, X.P. Su, and J.M. Toguri: CALPHAD, 2001, vol. 25, pp. 267–77.
N.Y. Tang, X.P. Su, and X.B. Yu: J. Phase Equilib., 2003, vol. 24, pp. 528–32.
X.H. Tang, F.C. Yin, X.M. Wang, J.H. Wang, X.P. Su, and N.Y. Tang: J. Phase Equilib., 2007, vol. 28, pp. 355–61.
N.Y. Tang and X.P. Su: Metall. Mater. Trans. A, 2002, vol. 33A, pp. 1559–61.
G. Reumont, G. Dupont, and P. Perrot: Z. Metallkd., 1995, vol. 86, pp. 608–13.
G. Reumont, M. Mathon, R. Fourmentin, and P. Perrot: Z. Metallkd., 2003, vol. 94, pp. 411–18.
Y.X. Liu, F.C. Yin, H. Tu, Z. Li, J. Wang, and X.P. Su: J. Phase Equilib., 2008, vol. 29, pp. 493–99.
Z.H. Wang, J.H. Wang, Y.H. Liu, N.Y. Tang, and X.P Su: J. Phase Equilib., 2006, vol. 27, pp. 469–76.
G. Reumont, T. Gloriant, and P. Perrot: J. Mater. Sci. Lett., 1997, vol. 16, pp. 62–65.
S.W. Pan, F.C. Yin, M.X. Zhao, Y. Liu, and X.P. Su: J. Alloys Compd., 2009, vol. 470, pp. 600–05.
H. Okamoto and L.E. Tanner: Bull. Alloy Phase Diagrams, 1990, vol. 11, pp. 377–84.
E. Wachtel and K. Tsiuplakis: Z. Metallkd., 1967, vol. 58, pp. 41–45.
J. Schramm: Z. Metallkd., 1940, vol. 32, pp. 399–407.
E.V. Potter and R.W. Huber: Trans. ASM, 1949, vol. 41, pp. 1001–22.
W.B. Henderson and R.J.M. Willcox: Philos. Mag., 1964, vol. 9, pp. 829–46.
O. Romer and E. Wachtel: Z. Metallkd., 1971, vol. 62, pp. 820–25.
Y. Nakagawa and T. Hori: Trans. Jpn. Inst. Met., 1972, vol. 13, p. 167.
H.H. Xu, X. Xiong, L.J. Zhang, Y. Du, and P.S. Wang: Metall. Mater. Trans. A, 2009, vol. 40A, pp. 2042–47.
M. Stange, H. Fjellvåg, S. Furuseth, and B.C. Hauback: J. Alloys Compds., 1997, vol. 259, pp. 140–44.
M. Elding-Pontén, L. Stenberg, A.K. Larsson, S. Lidin, and K. Ståhl: J. Solid State Chem., 1997, vol. 129, pp. 231–41.
Z. Moser, J. Dutkiewicz, W. Gasior, and J. Salawa: Bull. Alloy Phase Diagrams, 1985, vol. 6, pp. 330–34.
P.-E. Werner, L. Eriksson, and M. Westdahl: J. Appl. Cryst., 1985, vol. 18, pp. 367–70, http://www.ccp14.ac.uk/ccp/ccp14/ccp14-by-program/treor/ccp14-expanded/treor90-dos/
A. Boultif and D. Louër: J. Appl. Crystallogr., 2004, vol. 37, pp. 724–31.
J. Rodríguez-Carvajal: FullProf, http://www-llb.cea.fr/fullweb/powder.htm, 2009.
A.V. Tkachuk, L.G. Akselrud, Y.V. Stadnyk, and O.I. Bodak: J. Alloys Compds., 2000, vol. 312, pp. 284–87.
A.V. Tkachuk, Y. K. Gorelenko, Y. V. Stadnyk, and O.I. Bodak: J. Alloys Compds., 2001, vols. 317–318, pp. 280–83.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant Nos. 50721003 and 50831007), the Guangxi Science Foundation (Contract Nos. 0448022, 0540009, and 0640040), Guangxi Large Scale Apparatus Corporation Office, and the National Outstanding Youth Science Foundation of China (Grant No. 50425103). Thanks are also due to Mrs. J.R Huang for SEM/EDS examination.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 5, 2008.
Rights and permissions
About this article
Cite this article
Liang, J., Du, Y., Zhao, Q. et al. Determination of the Phase Equilibria in the Mn-Sn-Zn System at 500 °C. Metall Mater Trans A 40, 2909–2918 (2009). https://doi.org/10.1007/s11661-009-0026-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-009-0026-8