Abstract
The purpose of this work was to apply V and D cryo-plate methods for cryopreservation of Vaccinium corymbosum ʻToroʼ, Fragaria × ananassa ʻCleryʼ, and Amelanchier alnifolia (Nutt.) M. Roem. and to monitor the multiplication capacity of shoots regenerated from cryopreserved explants. Shoot tips pre-cultured for 1 d at 23°C in the dark on medium containing 0.3 M sucrose were used as explants. Loading was performed in a solution containing 2 M glycerol and 0.8 M sucrose (30 min at room temperature). In the V cryo-plate, dehydration was carried out at room temperature (20 to 50 min) using the following plant vitrification solutions: original PVS2, 90% PVS2 solution, and PVS3. Regarding the D cryo-plate, dehydration was performed in closed glass containers over silica gel for 2, 2.5, or 3 h. In both protocols, rewarming was carried out in a 1.0 M sucrose solution (15 min at 25°C). Regenerated shoots were multiplied and multiplication parameters were monitored after the second subculture. Using the V cryo-plate method, the highest regrowth in highbush blueberry was obtained following 50-min treatment with all three VSs (61.7 to 80.9%). The D cryo-plate method was even more suitable with maximum regrowth of 89.4% achieved after 2.5 h of desiccation. For strawberry, 62.5% was the highest regrowth recorded using PVS3-based V cryo-plate method while 83.3% of regrowth was observed using D cryo-plate protocol. Regrowth of saskatoon reached a maximum of 50% after 50-min treatment with PVS3 while it did not exceed 40% in other treatments. By the second subculture, shoots regenerated from cryopreserved explants regained and even exceeded the multiplication capacity of shoots regenerated from non-cryopreserved explants. This study is the first to present the successful application of the V cryo-plate method in highbush blueberry, as well as the utilization of both V and D cryo-plate methods in saskatoon.
Similar content being viewed by others
Introduction
There has always been a demand for alternative crops with unique nutritional traits. Small fruits, or “berries” for short, are small- to medium-sized fruits growing on perennial herbs, vines, or shrubs (Debnath 2003). Berries belong to the group of economically important crops, mainly for being a valuable and natural source of many biologically active compounds, such as vitamins, antioxidants, flavonoids, and anthocyanins (Moyer et al. 2002; Castrejón et al. 2008). Vaccinium spp. and Fragaria spp. are among the berries known worldwide, while Amelanchier alnifolia (Nutt.) M. Roem. is grown regionally (Ochmian et al. 2013).
A. alnifolia (Nutt.) M. Roem. (saskatoon) is a fruit-bearing shrub native to the Northern Hemisphere that is attractive for its frost and disease resistance and decorative purposes. Its fruit quality is very similar to blueberries, and the ripe berries contain high levels of vitamins C and B12 and essential minerals (Mazza 2005). Vaccinium corymbosum (highbush blueberry) is an economically important small fruit that originated in North America and has also been successfully introduced into Europe (Fira et al. 2008). The dark-colored fruits contain a significant amount of anthocyanins, which are believed to have chemopreventive effects, and flavonoids, which act as antioxidants (Castrejón et al. 2008; Aqil et al. 2016). Fragaria × ananassa (strawberry) is a well-known and widely appreciated berry, mainly for its bright red color, juicy texture, and characteristic aroma (Ashrafuzzaman et al. 2013). It is best known for its remarkably high vitamin C and folate content and is one of the most widely consumed berries, either fresh or processed into juices, jams, and syrups (Giamperi et al. 2014).
Cryopreservation is an ideal method for long-term storage of germplasm since preservation of clonal genotypes of most fruit crops requires time-consuming vegetative propagation. Plant material can be stored without modification for a theoretically unlimited period of time, is protected from contamination, and requires very little maintenance (Engelmann 2004; Reed 2008). Cryopreservation serves various purposes (Panis and Thinh 2001). It facilitates hybridization between plants with differing flowering schedules and allows for hybridization between geographically separated plant populations. Furthermore, cryopreservation aids in reducing the transmission of diseases through pollination vectors. Importantly, it minimizes the risk of somaclonal variation, typically encountered in tissue culture, and gametoclonal variation, which occurs in field collections. Consequently, this method enables the establishment of cultures characterized by both vegetative propagation and genetic uniformity among clones (Roque-Borda et al. 2021).
Large-scale use of cryogenic storage in small fruit research is hampered by genotype-specific responses to cryoprotective treatments. Thus, there is a need to develop protocols for lesser-known species and optimize existing protocols as well as to use promising techniques, such as V- and D cryo-plate (Niino et al. 2013; Yamamoto et al. 2011). These are less laborious and result in enhanced regrowth potential compared to the conventional cryogenic methods (Matsumoto 2017). As a result, high regrowth percentages are anticipated following cryopreservation.
To date, vitrification-based cryopreservation methods had been used in most cases for cryopreservation of highbush blueberry and strawberry. For V. corymbosum, protocols for encapsulation-dehydration (Uchendu and Reed 2009), vitrification (Kami et al. 2009), and droplet-vitrification (Wang et al. 2017) have been successfully applied. No V cryo-plate developed protocol has been described for this species so far, although some researchers have already applied this method to other Vaccinium spp., such as V. ashei and V. virgatum (Matsumoto et al. 2014; Takimoto et al. 2020). The D cryo-plate method was used so far in only one study focused on five V. corymbosum cultivars (Dhungana et al. 2017). For F. ananassa, methods for encapsulation-dehydration (Höfer and Reed 2010), vitrification (Niino et al. 2003), and droplet-vitrification (Pinker et al. 2009) have already been developed. Cryo-plate methods are less applied for this species. Yamamoto et al. (2012) used the V cryo-plate method for cryopreservation of shoot tips of 15 strawberry cultivars with an average regrowth of 81%, while the D cryo-plate method was described so far in a study focused on F. ananassa cultivar ʻEarliglowʼ with maximum regrowth of 40% (Gupta and Tewari 2020). In the case of A. alnifolia (Nutt.) M. Roem., no cryopreservation protocol has been developed yet.
This work was undertaken in response to the strikingly limited number of published studies on highbush blueberry and strawberry, and the notable absence of any published results related to saskatoon when considering the application of cryo-plate methods. The study aims to investigate the efficiency of V cryo-plate and D cryo-plate methods in successfully cryopreserving and regrowing these three small fruit cultivars. In addition, the study evaluates the multiplication index as well as the length of axial and axillary shoots regenerated from cryopreserved explants. This investigation represents a novel approach as, to the best of present authors’ knowledge, the multiplication capacity of post-cryopreserved shoots has not been previously studied in these species. The findings will provide valuable insights for determining the most suitable method for each cultivar and understanding the impact of cryopreservation on the regenerative potential of treated plants.
Material and Methods
Plant Material and Establishment of Aseptic Culture
Highbush blueberry cultivar ʻToroʼ (V. corymbosum), strawberry cultivar ʻCleryʼ (F. ananassa), and saskatoon (A. alnifolia (Nutt.) M. Roem.) were included in the experiments. Aseptic cultures of highbush blueberry and saskatoon were initiated at the Institute of Plant Genetics and Biotechnology SAS, Nitra, Slovakia, while that of strawberry was established at the Fruit Research Institute, Čačak, Serbia. All cryopreservation experiments were performed in the Tissue Culture Laboratory of Fruit Research Institute Čačak.
Highbush blueberry and saskatoon sprouts bearing several axillary or apical buds were taken from mature plants, cut into smaller pieces (1 to 1.5 cm), and sterilized with 70% (v/v) ethanol for 2 min followed by immersion in 0.1% (w/v) HgCl2 (Sigma-Aldrich, St. Louis, MO) with a few drops of Tween 20 (Sigma-Aldrich) for 5 min along with three rinses in sterile distilled water. In strawberry, runner tips (1 to 2 cm) were excised from mother plants during the growing season in the greenhouse. Disinfection was performed by immersing in 70% ethanol for 1 min and then in 1.0% (v/v) NaOCl (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) for 15 min followed by triple rinsing in sterile distilled water. Following sterilization, single-node explants of highbush blueberry and saskatoon and shoot tips excised from strawberry runners were placed in sterile Petri dishes (6 cm in diameter) filled with the culture medium. In vitro donor cultures were established on full-strength Murashige and Skoog medium (MS, Murashige and Skoog 1962, Duchefa Biochemie B.V., Haarlem, NL) for strawberry and saskatoon and on medium with macro-salts reduced to ½ in highbush blueberry. All media were supplemented with 30.0 g L−1 sucrose (Slavus, Bratislava, SK) and 8.0 g L−1 plant agar (Duchefa Biochemie). The pH of the media was adjusted to 5.6 (full-strength MS) or 4.8 (MS with half-strength macro-salts) before autoclaving for 20 min at 121°C and 120 kPa. All plant growth regulators (PGR) were filter-sterilized before addition to the culture medium. Growth regulators (all from Duchefa Biochemie) for shoot initiation were used as follows: 1.0 or 2.0 mg L−1 6-benzylaminopurine (BAP) combined with 0.5 mg L−1 indole-3-butyric acid (IBA) for saskatoon (Hunková et al. 2021) and strawberry (Ashrafuzzaman et al. 2013), respectively, and 2.0 mg L−1 zeatin with 0.2 mg L−1 indole-3-acetic acid (IAA) for highbush blueberry, respectively (Hunková et al. 2023). After initiation, shoots from each genotype were subcultured at 4-wk intervals on medium with constant PGR composition to obtain a sufficient number of mother stock axillary shoots for cryopreservation experiments. All cultures were maintained in a growth chamber at 23 ± 1°C with a 16-h light/8-h dark photoperiod under cool white-fluorescent lights at a photosynthetic photon flux density 50 μmol m−2 s−1 (Alfaco inženjering d.o.o., Čačak, Serbia).
Explant Isolation and Pretreatment
Shoot tips (1.5 mm long) excised from 6-wk-old plantlets grown in vitro were used as initial explants for cryopreservation experiments. After dissection, explants were grown for 24 h in the dark at 23°C on a solid multiplication medium supplemented with 0.3 M sucrose. For highbush blueberry, MS with macro-salts reduced to ½ (micro-salts and vitamins unchanged, Duchefa Biochemie B.V.) with 2.0 mg L−1 zeatin, 0.2 mg L−1 IAA, and pH adjusted to 4.8 was used as the basal medium. For strawberry and saskatoon, the basal medium was a full-strength MS medium with 1.0 mg L−1 BA, 0.1 mg L−1 IBA, and 0.1 mg L−1 gibberellic acid (GA3) with 0.3 M sucrose and pH set to 5.6. The stated PGR combination was previously determined as the most suitable in the multiplication stage of tested genotypes (data are not presented).
Cryopreservation
Aluminum cryo-plates (7 mm × 37 mm × 0.5 mm; Taiyo Nippon Sanso Corp., Tokyo, JP) that fit into 2.0-mL cryotubes with 10 or 12 oval-shaped wells [2.5 mm (L) × 1.5 mm (W) × 0.75 mm (D)] were used as explants carriers (Yamamoto et al. 2011) in both V cryo-plate and D cryo-plate protocol. Excised shoot tips were plated in wells of aluminum cryo-plate filled with approximately 4.0 μL of 2% (w/w) Na-alginate (Sigma-Aldrich) solution in a calcium-free basal medium containing 0.4 M sucrose. Then, 0.1 M CaCl2 (Carl Roth GmbH + Co. KG) solution supplemented with 0.4 M sucrose in the basal medium was poured on the aluminum plates with shoot tips and left for 30 min for polymerization. After removing the CaCl2 solution by gently tapping onto filter paper, the cryo-plates with attached shoot tips were treated with a loading solution (LS) containing 2 M glycerol (Carlo Erba Reagents srl, Cornaredo MI, IT) and 0.8 M sucrose. Osmoprotection was performed for 30 min at room temperature.
In the protocol for V cryo-plate, explants were dehydrated at room temperature with original PVS2 solution (13.7% sucrose, 30.0% glycerol, 15.0% ethylene glycol (EG, Carlo Erba Reagents srl), and 15% dimethylsulfoxide (DMSO, Carlo Erba Reagents srl)) (Sakai et al. 1990); 90% PVS2 solution–PVS A3 solution (22.5% sucrose, 37.5% glycerol, 15.0% EG, and 15.0% DMSO) (Kim et al. 2009); and PVS3 solution (50.0% glycerol and 50.0% sucrose) (Nishizawa et al. 1993). Highbush blueberry shoot tips were dehydrated for 20, 40, and 50 min with each vitrification solution (VS). Based on the best regrowth percentages in this genotype, 50-min treatment was chosen as the most efficient for all three VS types and was applied for cryopreservation of saskatoon and strawberry. The protocol for the D cryo-plate method consisted of the same steps as the V cryo-plate procedure except for the dehydration step, which was performed by desiccation in closed 100-mL glass containers over 40.0 g of silica gel (Carl Roth GmbH + Co. KG) at 23°C for 2, 2.5, and 3 h. After dehydration or desiccation, cryo-plates with shoot tips were transferred to uncapped 2.0-mL cryotubes (Carl Roth GmbH + Co. KG) held on a cryo-cane and directly immersed in liquid nitrogen (LN) for 1 h.
For rapid warming and unloading, cryo-plates with shoot tips were transferred directly from LN to 1 M sucrose solution for 15 min at 25°C. Then, shoot tips were placed on solidified regeneration medium (corresponds to those determined to be most suitable in the multiplication stage). Shoots were cultivated in the dark for 7 d and then under standard conditions in the growth room (23 ± 1°C; 16-h light/8-h dark photoperiod; 50 μmol m−2 s−1 light intensity).
Assessment of Growth Recovery
Regrowth was specifically defined as the regeneration of viable shoots from the shoot tips within the first 8 wk of cultivation. Regrowth percentages were expressed relative to the total number of shoot tips treated. The mean number of regenerated shoots per explant was determined by dividing the total number of obtained shoots by the number of explants that showed regeneration in each treatment.
Three independent replicates of approximately 12 shoot tips per each replicate were tested in each experimental treatment for each individual genotype. Therefore, 972 explants were isolated for highbush blueberry (27 treatments × 3 replicates × 12 explants) while 540 explants were isolated per genotype for strawberry and saskatoon (15 treatments × 3 replicates × 12 explants per each genotype). If the shoot tips were treated with LN for at least 1 h, they were considered as cryopreservation treatments and referred to as “ + LN.” When treatments did not include the cooling explants in LN, they were considered controls and labeled as “ − LN.” Therefore, the experiments included the following controls: dissection control, pregrowth control, loading control, dehydration, and desiccation control.
Monitoring of Shoot Multiplication Parameters
After regeneration, shoots derived from different treatments were transplanted separately onto a multiplication medium (previously defined for each genotype as the most suitable for multiplication), labeled according to their origin, and subcultured for two consecutive cycles (each lasting 28 d) on a medium with constant composition. At the end of the second subculture, multiplication parameters including multiplication index and length of axial and axillary shoots were determined. Multiplication index was defined as the number of newly formed shoots (longer than 5 mm) per initial shoot planted at the beginning of the second subculture. All parameters were measured on at least 30 randomly selected plantlets of different origins in each genotype (three replicates with at least of 10 plantlets per replicate).
Statistical Analysis
For comparison and determination of significant differences among means (P ≤ 0.05), one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test was performed using Statistica 12.0 package for Windows (StatSoft, Inc., Tulsa, OK). Data presented as percentages were subjected to an arcsine transformation before ANOVA to stabilize the variance of the data.
Results
Cryopreservation of Highbush Blueberry
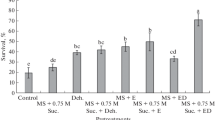
Concerning regeneration, the highest percentage of regrowth was observed in the controls for dissection, pretreatments, and loading. However, the other treatments (dehydration, desiccation, and cryopreservation) generally resulted in a decrease of shoot regeneration ability for both applied methods (Table 1). Comparison of dehydration controls with the corresponding cryopreserved explants showed that LN exposure significantly affected the regenerative capacity of the explants. The percentages of regeneration in PVS2 dehydration controls were above 90%, whereas in cryopreserved explants, these percentages were significantly lower and varied across a broad range, depending on the treatment duration. The highest percentage of regeneration for cryopreserved explants was observed in the 50-min PVS2 treatment (Fig. 1a). Dehydration with PVS A3 solution also resulted in high regeneration percentages (above 90%) for control samples and significantly lower values for corresponding cryopreserved explants. The best regrowth of cryopreserved explants (around 80%) was achieved also with the 50-min treatment (Fig. 1b). PVS3 dehydration exhibited varying regenerative trends for controls, with the highest regrowth rates observed for these samples. On the other hand, cryopreserved explants displayed a range of regrowth percentages, with the 50-min PVS3 treatment yielding the most favorable outcome—above 75% (Fig. 1c). In contrast, the D cryo-plate method protocol resulted in a less significant reduction in regenerative capacity after cryopreservation, with similar regeneration percentages observed for desiccation controls and cryopreserved explants (Fig. 1d–f). Very high regrowth percentages (approximately 80% or higher) were consistently attained in cryopreserved explants, irrespective of the desiccation duration (Table 1).
Regrowth of highbush blueberry Vaccinium corymbosum (Ciocârlan 1990) ‘Toro’ shoot tips cryopreserved by V cryo-plate method—explants dehydrated for 50 min at room temperature using PVS2 (a), PVS A3 (b), and PVS3 (c); and D cryo-plate method—explants desiccated for 2 h (d), 2.5 h (e), and 3 h (f). The diameter of Petri dish was 5.5 cm. PVS2—plant vitrification solution comprising 13.7% sucrose, 30.0% glycerol, 15.0% ethylene glycol, and 15.0% dimethylsulfoxide; PVS A3—90% PVS2 (22.5% sucrose, 37.5% glycerol, 15.0% ethylene glycol, and 15.0% dimethylsulfoxide); PVS3—plant vitrification solution comprising 50.0% glycerol and 50.0% sucrose.
The average number of regenerated shoots per explant ranged from 1 to 2.4 in the V cryo-plate protocol and from 1.3 to 2 in the D cryo-plate protocol with no significant differences observed between cryopreserved explants and the corresponding dehydration and desiccation controls (Table 1). Shoots regenerated from explants after both V and D cryo-plate methods were small but green and healthy without any chlorosis or necrosis (Fig. 1).
Cryopreservation of Strawberry and Saskatoon
Based on the best results obtained with the V cryo-plate method for highbush blueberry cryopreservation, the 50-min treatments with PVS2, PVS A3, and PVS3 were selected for cryopreservation experiments in strawberry and saskatoon (Tables 2 and 3). As for the D cryo-plate method, all three desiccation times were tested.
In strawberry, all steps following pregrowth treatment (loading, dehydration, desiccation, and cryopreservation) significantly reduced the percentage of shoot regeneration for both methods (Table 2). However, comparing the dehydration and desiccation controls with the corresponding cryopreserved explants, it was found that exposure to LN had no significant effect on explant regrowth in most treatments. A significant decrease in regenerative capacity was observed only in PVS2 treatment (Table 2). In the D cryo-plate method protocol, regeneration in control samples consistently exceeded 60% across all treatments and exhibited a narrower range of variation compared to the corresponding cryopreserved explants, where regrowth ranged from just above 45% to slightly over 80% (Table 2). Overall, the highest regrowth of cryopreserved explants was achieved with PVS3 dehydration and after 2.5 h of desiccation. The number of regenerants per explant in the V cryo-plate protocol ranged from 3 to 5.3 for dehydration controls and 2.7 to 4 for cryopreserved explants. Lower values of this parameter were recorded in the D cryo-plate protocol, ranging from 2.3 to 2.6 for desiccation controls and 2.5 to 3.8 for cryopreserved explants. Compared with pregrowth and loading controls, these values were not statistically different (Table 2). Shoots regenerated from explants cryopreserved by the V cryo-plate method were well developed and had a large number of bright green, large leaves showing signs of hyperhydricity (Fig. 2a–c). Shoots regenerated from explants cryopreserved using the D cryo-plate method were less vigorous, with smaller leaves, but without signs of hyperhydricity (Fig. 2d–f).
Regrowth of strawberry Fragaria × ananassa ‘Clery’ shoot tips cryopreserved by V cryo-plate method—explants dehydrated for 50 min at room temperature using PVS2 (a), PVS A3 (b), and PVS3 (c); and D cryo-plate method—explants desiccated for 2 h (d), 2.5 h (e), and 3 h (f). The diameter of Petri dish was 5.5 cm. PVS2—plant vitrification solution comprising 13.7% sucrose, 30.0% glycerol, 15.0% ethylene glycol, and 15.0% dimethylsulfoxide; PVS A3—90% PVS2 (22.5% sucrose, 37.5% glycerol, 15.0% ethylene glycol, and 15.0% dimethylsulfoxide); PVS3—plant vitrification solution comprising 50% glycerol and 50% sucrose.
In saskatoon, the highest regrowth was also observed in the dissection, pregrowth, and loading controls while the other treatments (dehydration, desiccation, and cryopreservation) reduced the percentage of shoot regeneration in most cases (Table 3). Using the V cryo-plate method, the lowest regeneration percentage in control samples was 50%, and it reached 100% in the case of PVS3 treatment, while regrowth in cryopreserved explants consistently remained at 50% or lower, with no regrowth observed in the case of PVS2 treatment (Fig. 3a–c). PVS3 dehydration resulted in the highest percentage of regeneration of cryopreserved shoot tips. The D cryo-plate method proved to be significantly less effective in cryopreserving this genotype. The regrowth percentages were low in both desiccation controls and the corresponding cryopreserved explants (Fig. 3d–f), with the highest value being achieved after 2 h of desiccation. The average number of regenerated shoots per cryopreserved explant ranged from 2.5 to 3.5 in the V cryo-plate protocol and from 1 to 1.1 in the D cryo-plate protocol (Table 3). Shoots regenerated from explants after the V cryo-plate method with PVS A3 and PVS3 were vigorous and green with no signs of hyperhydricity. However, almost no shoots were observed after D cryo-plate treatment (Fig. 3).
Regrowth of saskatoon Amelanchier alnifolia (Nutt.) M. Roem. (Wisskirchen et al. 1998) shoot tips cryopreserved by V cryo-plate method—explants dehydrated for 50 min at room temperature using PVS2 (a), PVS A3 (b), and PVS3 (c); and D cryo-plate method—explants desiccated for 2 h (d), 2.5 h (e), and 3 h (f). The diameter of Petri dish was 5.5 cm. PVS2—plant vitrification solution comprising 13.7% sucrose, 30.0% glycerol, 15.0% ethylene glycol, and 15.0% dimethylsulfoxide; PVS A3—90% PVS2 (22.5% sucrose, 37.5% glycerol, 15.0% ethylene glycol and 15.0% dimethylsulfoxide); PVS3—plant vitrification solution comprising 50.0% glycerol and 50.0% sucrose.
Multiplication of Regenerated Shoots
To evaluate the effect of each step in the applied cryopreservation protocols on the multiplication capacity of regenerated shoots in the second subculture after regrowth, a one-way ANOVA was used. The index of multiplication and the length of axial and lateral shoots were determined in shoots of different origins for each of the three genotypes studied (Table 4).
When compared with the dissection controls (shoot tips placed on regrowth medium directly after dissection), both pregrowth and loading treatments resulted in a slight decrease in multiplication capacity of regenerated shoots in highbush blueberry and saskatoon. Contrary results were observed in strawberry where a significantly higher value of this parameter was determined in shoots regenerated from loading controls compared to those originating from dissection controls. The 50-min dehydration of highbush blueberry and strawberry explants with PVSs mostly had no significant effect on the multiplication of regenerated shoots. However, an exception was the PVS A3 treatment, which resulted in a significant increase in the multiplication index of highbush blueberry shoots regenerated from cryopreserved explants. Moreover, this treatment resulted in a significant decrease in the parameter of strawberry shoots originating from both control and cryopreserved explants. Similarly, in saskatoon, a significant decrease in the multiplication capacity of shoots regenerated from explants dehydrated with all VSs (both control and cryotreated) was observed. In terms of desiccation, this step in the D cryo-plate procedure caused a significant decrease in the multiplication capacity of both control and cryopreserved shoots in all genotypes.
Lengths of axial and lateral shoots varied significantly with the highest and lowest values observed in shoots regenerated from dehydrated, desiccated explants. In highbush blueberry, the longest axial and lateral shoots were measured in those regenerated from PVS3-dehydrated explants, while the shortest shoots were obtained from cryopreserved explants desiccated for 3 h. In strawberry, dehydration of both control and cryopreserved explants with VSs resulted in regeneration of significantly longer axial and lateral shoots compared with those originated from explants desiccated over silica gel. However, completely opposite results were observed in saskatoon where the longest axial and lateral shoots were observed in plantlets regenerated from cryopreserved explants desiccated for 3 h.
Discussion
Modern cryopreservation techniques rely on vitrification, which involves rapid cooling of plant material to a temperature below the glass transition point, at which intracellular and extracellular water transitions into an amorphous, glass-like state without the formation of damaging ice crystals (Benson 2008). Therefore, the critical step for successful recovery growth in vitrification-based methods is the dehydration process (Sakai and Engelmann 2007), which can be achieved by exposing samples to vitrification solutions or by air dehydration at temperatures above freezing. Among the various vitrification-based cryotechniques that have been successfully applied to numerous plant species (Engelmann 2014; Matsumoto and Niino 2014), droplet-vitrification (Panis et al. 2005) and cryopreservation using cryo-plates (Yamamoto et al. 2011) allow for rapid cooling and rewarming rates that can minimize the cell exposure to extreme temperatures and reduce the potential for cellular damage. As a result, they enable higher post-rewarming recovery compared to some other cryopreservation methods.
Dehydration with highly concentrated VSs is one of the most critical steps in developing any vitrification-based cryopreservation protocol. The V cryo-plate method was designed to streamline the cryopreservation process by attaching plant explants to a reusable aluminum carrier that reduces the need for excessive handling, minimizes loss of plant material, and allows precise control over the timing of dehydration (Niino et al. 2019). To the present authors’ knowledge, there are no previous reports on the use of the V cryo-plate method for cryopreservation of in vitro shoot tips of V. corymbosum. Matsumoto et al. (2014) utilized a PVS2-based V cryo-plate method for cryopreservation of dormant buds in ten V. virgatum cultivars. The study reported the highest regrowth of 84% with the 30-min treatment. The results in the present study demonstrated the feasibility of 50-min treatments with PVS2, PVS A3, and PVS3, which resulted in significant increase in regrowth success of cryopreserved explants with maximum over 80%. Determining the optimal duration of exposure to VSs is crucial to achieve the right balance between toxicity and sufficient dehydration of explants, thereby minimizing the risk of ice crystal formation in cryopreserved tissues (Panis et al. 2005). In the current study, dehydration controls for the 20- and 40-min treatments with all three VSs also exhibited a high regrowth potential, exceeding 90% for most treatments; however, corresponding cryopreserved explants had significantly lower regeneration capacity, particularly for the 20-min treatment; Uchendu and Reed (2009) employed the PVS2-based vitrification technique for the cryopreservation of shoot tips of cultivars ʻBerkeleyʼ, ʻO’Nealʼ, and ʻBrigitta Blueʼ (V. corymbosum). In their research, 20-min dehydration at 25℃ using this VS enabled regrowth ranging from 33 to 87% with the highest regrowth observed in the ʻBrigitta Blueʼ. In the present study, greater resistance of ʻToroʼ to the chemical toxicity of the PVS2 solution at 25℃ was observed, and the success of viable shoot regeneration increased significantly with prolonged dehydration up to treatment duration of 50 min. It is worth noting that in the present study, the previous preconditioning of mother plants and preculture of isolated shoot tips were not applied as performed in the experiments by Uchendu and Reed (2009). In a separate study conducted by Wang et al. (2017) that focused on PVS2-based droplet-vitrification of various V. corymbosum cultivars, they achieved regrowth percentages ranging from 47 to 91%. The authors determined that a treatment duration of 40 min was optimal, which is shorter than the 50-min treatment duration determined as the most suitable in the present experiment. Although tolerance to chemical toxicity of vitrification solutions may be genotype-dependent, the increased tolerance to prolonged PVS2 treatment observed in the present experiment may be due to the possible mitigation of chemical toxicity by the calcium alginate gel used to attach shoot tips to aluminum plates (Yamamoto et al. 2011).
Comparing the VSs among themselves, it can be concluded that PVS A3- and PVS3-based dehydration were superior to PVS2 dehydration in highbush blueberry ʻToroʼ. In addition, markedly lower proliferation capacity of shoots regenerated from control and cryopreserved explants treated with PVS2 compared with PVS A3 and PVS3 dehydrated shoot tips indicates that this genotype is more sensitive to the cytotoxicity of this VS. This agrees with previous studies of present authors focused on droplet-vitrification technique used for cryopreservation of blackberry ‘Čačanska Bestrna’ (Vujović et al. 2011, 2015a). Kim et al. (2009) demonstrated that a balanced composition of PVS A3 solution can be more beneficial for the recovery of cryopreserved samples compared to the original PVS2 solution, while PVS3 solution is recommended to use for samples sensitive to chemical toxicity and tolerant to osmotic stress. To the present authors’ knowledge, no other study has compared the effect of PVS A3 and PVS3 on highbush blueberry cryopreservation.
Considering that the 50-min treatment with all three VSs resulted in the highest regrowth of highbush blueberry ʻToroʼ (Ericaceae family), the present authors chose this treatment for further experiments with strawberry and saskatoon (both belonging to the Rosaceae family). This decision was made despite the recommendation that the type or modifications of VSs should be chosen from cryoprotective solutions that have shown high regeneration in species of the same family (Zamecnik et al. 2021).
Regarding the strawberry ʻCleryʼ, no significant difference in regrowth was observed between explants treated with the original PVS2 solution and PVS A3 solution. These results (regrowth around 50%) were notably lower than those obtained by Yamamoto et al. (2012), who achieved an average regrowth level of 81% in 15 strawberry cultivars with the V cryo-plate method using PVS2 solution for 50 min. The higher regrowth percentages observed in their study can be attributed to the improved physiological state of the donor plants achieved by preconditioning, which involved cold accumulation at 5°C for 3 to 4 wk. In an effort to simplify and speed up the cryopreservation procedure, the present authors attempted to omit the cold acclimation step and proceed directly to cryopreservation by using shoot tips isolated from plants grown under standard conditions. In the present research, the preculturing of strawberry shoot tips on agar-solidified medium with 0.3 M sucrose was probably not sufficient to achieve as high regrowth percentages after cryopreservation as those reported by Yamamoto et al. (2012). The importance of cold acclimation in achieving high regrowth levels after cryopreservation using other vitrification-based cryopreservation techniques in strawberries has already been emphasized by Reed and Hummer (1995) and Niino et al. (2003). However, the present results are comparable to those achieved in three strawberry cultivars (regrowth ranged between 40 and 67%) cryopreserved using the PVS2-based droplet-vitrification technique (Pinker et al. 2009), although the duration of VS treatment was twice as long in the present study. PVS3-based dehydration for 50 min in strawberry ʻCleryʼ resulted in significantly higher regrowth (above 60%) compared to that obtained with PVS2 and PVS A3. Similarly, Bae et al. (2021) reported average regrowth of 61.5% in 26 strawberry accessions cryopreserved using the PVS3-based droplet-vitrification protocol.
The lowest regeneration potential using the V cryo-plate method was observed in saskatoon where the regrowth reached less than 40% under dehydration with PVS2 and PVS A3 while no regrowth was observed after the use of PVS2. These results along with significant decrease in regrowth percentage of corresponding dehydration controls compared to dissection, pregrowth, and loading controls indicated that this genotype is highly sensitive to biochemical toxicity of PVS2 or its variants. Therefore, the present authors recommend that treatment should be shorter than 50 min or performed at 0°C to mitigate the detrimental effects of these VSs. On the other hand, this genotype showed tolerance to the osmotic toxicity of PVS3 solution, as evidenced by the maximum recovery of control explants and a regrowth of 50% in cryopreserved explants. Although there are no available results for saskatoon from other studies, other species from the Rosaceae family have been cryopreserved using various vitrification-based cryopreservation techniques. In a study conducted by Vujović et al. (2020), the percentage of regrowth using PVS A3-based droplet-vitrification increased significantly when dehydration was performed on ice (45 to 70%) compared with dehydration at room temperature (0 to 14.5%) for the same treatment duration (30 to 50 min). Regrowth of PVS3-treated shoot tips (40 to 60 min) showed no significant differences (45 to 50%), which is comparable to the present study’s results for saskatoon. Droplet-vitrification was also used in the studies by Pawłowska and Szewczyk-Taranek (2014) and Le Bras et al. (2014) for cryopreservation of shoot tips and buds taken from in situ plants of Rosa spp., achieving regrowth percentages up to 87% in both studies.
The D cryo-plate method was developed to address the challenges posed by the high sensitivity of certain plants to PVS2 treatment, replacing the VS treatment with air dehydration (Niino et al. 2014). In the present research, efficient regeneration was achieved by using this method in highbush blueberry (up to 89.4%) as well as in strawberry (up to 83.3%) with the highest regrowth percentage observed after 2.5 h of desiccation in both genotypes. These results are similar to those reported for blueberry ʻTifblueʼ (V. ashei J. M. Reade), which were approximately 90% (Dhungana et al. 2017). The authors of that study emphasized the optimization of blueberry cryopreservation, using preculture and loading treatment conditions very similar to experiments in the current study. The only difference was in the desiccation process, which, in their study, was carried out under the airflow of a laminar flow cabinet, as opposed to being conducted in closed glass containers over silica gel. Uchendu and Reed (2009) utilized the encapsulation-dehydration technique for cryopreserving highbush blueberry; and after 6 h of desiccation, at least 80% of the encapsulated shoot tips successfully developed into shoots in all cultivars studied. However, comparable results were achieved in the present study with much shorter desiccation periods using the D cryo-plate method without any preconditioning of mother plants in vitro. With respect to strawberries, the outcomes in the current study surpassed the 40% maximum regrowth reported by Gupta and Tewari (2020) when utilizing the D cryo-plate protocol for ‘Earliglow’ strawberry. This suggests that the efficiency of post-rewarming regeneration is probably cultivar-specific. In the study of Höfer and Reed (2010), shoot regeneration of two strawberry cultivars cryopreserved by encapsulation-dehydration reached up to 90% after 6 h of dehydration. Compared to these findings, the present study indicates that the D cryo-plate method is highly efficient for cryopreservation of F. ananassa ʻCleryʼ.
In contrast to highbush blueberry and strawberry, saskatoon again displayed much lower regrowth potential under the experimental conditions set up for D cryo-plate with the highest regrowth being around 25% for desiccation controls and 20% for cryopreserved specimens. The high sensitivity to chemical and physical dehydration suggested that the protocol for cryopreservation of this genotype should include preconditioning of mother plants for the acquisition of dehydration tolerance and thus achieving high levels of regrowth following cryopreservation. This requirement for cold-hardening as well as its optimal duration is species-specific (Niino et al. 2019). Moreover, the duration of sample dehydration directly affects the regrowth, as shown in Rosa chinensis L., where the 3- and 4-h treatments exceeded the 2-h treatment (Le Bras et al. 2014).
Cryopreservation exposes plant material to various stresses that can affect both cryopreserved specimens and regenerated plantlets (Reed et al. 2005). The development of an efficient and robust in vitro system enabling vigorous regrowth of explants retrieved from LN and rapid multiplication of regenerated shoots is of utmost importance in the cryopreservation process. Therefore, monitoring the multiplication capacity of shoots regenerated after cryopreservation is crucial for evaluating the success and effectiveness of cryopreservation protocols. It serves as a valuable indicator of the overall health and vigor of regenerated plantlets and their ability to undergo rapid clonal propagation. In the present research, plantlets regenerated from cryopreserved explants showed normal morphology and the ability to multiply. When comparing the shoots originating from cryopreserved explants with those regenerated from corresponding dehydration/desiccation controls, the present authors mostly did not observe significant differences in analyzed multiplication parameters. Interestingly, in some treatments, the cryopreserved shoots had significantly higher multiplication index and length than the corresponding control shoots (originated from explants that were desiccated or dehydrated but not cryopreserved). Moreover, in the case of highbush blueberry and strawberry, the multiplication capacity of cryopreserved shoots derived from the V cryo-plate protocol even surpassed that of plantlets regenerated from dissection controls (explants that did not undergo any treatment following dissection). Similar findings were reported for Prunus sp. shoots cryopreserved using V and D cryo-plate methods (Vujović et al. 2015b). However, in apple shoots regenerated from cryopreserved explants using droplet-vitrification, dehydration with VS resulted in a significant reduction in multiplication capacity, especially in terms of multiplication index. This reduction was observed in comparison to shoots derived from dissection and pregrowth controls (Vujović et al. 2020). Conversely, cryopreservation did not have an impact on the multiplication phase of olive cultures derived from somatic embryos (Bradaï and Sánchez-Romero 2021). However, there was a notable interaction between genotype and cryopreservation concerning shoot length during the multiplication process. This phenomenon, observed in plantlets recovered from cryopreserved explants, was probably stress-induced and species-specific. Leunufna and Keller (2021) also found no significant differences in multiplication capacity among untreated, PVS2-treated, and cryopreservation-derived in vitro plantlets in two of the three evaluated yam (Dioscorea spp.) species. However, for the third species, the untreated in vitro plantlets exhibited a significantly lower multiplication rate compared to dehydration controls and shoots originating from cryotreated explants. According to those authors, this difference could be attributed to physiological effects that are expected to recover in subsequent subculture cycles.
Conclusions
This study is the first to present the successful application of the V cryo-plate method in highbush blueberry, as well as the utilization of both V and D cryo-plate methods in saskatoon. Furthermore, multiplication of shoots regenerated from cryopreserved explants was successful in all examined cultivars with regenerative abilities exceeding even those of control shoots originating from non-cryopreserved explants. The preserved capacity for multiplication of cryopreserved shoots confirms the effectiveness of the employed cryopreservation methods. Therefore, both V and D cryo-plate can be recommended for highbush blueberry and strawberry, but only V cryo-plate should be used for saskatoon. Further research should focus on the evaluation of the rooting ability and acclimatization success of shoots originating from cryopreserved specimens. This will enable us to comprehensively assess the success of the cryopreservation process, ensuring that the preserved plant material retains its capacity to generate roots and establish itself in a new environment.
References
Aqil F, Jeyabalan J, Kausar H, Munagala R, Singh IP, Gupta R (2016) Lung cancer inhibitory activity of dietary berries and berry polyphenolics. J Berry Res 6:105–114. https://doi.org/10.3233/JBR-160120
Ashrafuzzaman M, Faisal SM, Yadav D, Khanam D, Raihan F (2013) Micropropagation of strawberry (Fragaria ananassa) through runner culture. Bangladesh J Agril Res 38:467–472
Bae J, Lee S-Y, Song J-Y, Lee J-R, Yoon M, Yi J-Y, Kim H-H, Lee Y-Y (2021) Efficient cryopreservation of in vitro grown shoot tips of strawberry (Fragaria x ananassa Duch.) germplasm using droplet-vitrification. Korean J Plant Res 34:600–607. https://doi.org/10.7732/kjpr.2021.34.6.600
Benson EE (2008) Cryopreservation of phytodiversity: a critical appraisal of theory & practice. Crit Rev Plant Sci 27:141–219. https://doi.org/10.1080/07352680802202034
Bradaï F, Sánchez-Romero C (2021) Effect of cryopreservation on the ex vitro establishment of olive plants regenerated via somatic embryogenesis. Plants 10:396. https://doi.org/10.3390/plants10020396
Castrejón ADR, Eichholz I, Rohn S, Kroh LW, Huyskens-Keil S (2008) Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem 109:564–572. https://doi.org/10.1016/j.foodchem.2008.01.007
Ciocârlan V (1990) Flora ilustrata a României, Pteridophyta et Spermatophyta 2. Editura Ceres, Bucharest, Romania
Debnath SC (2003) Micropropagation of small fruits. In: Jain SM, Ishii K (eds) Micropropagation of woody trees and fruits. Kluwer Academic Publishers, Dordrecht, pp 465–506
Dhungana SA, Kunitake H, Niino T, Yamamoto S, Fukui K, Tanaka D, Maki S, Matsumoto T (2017) Cryopreservation of blueberry shoot tips derived from in vitro and current shoots using D cryo-plate technique. Plant Biotechnol 34:1–5. https://doi.org/10.5511/plantbiotechnology.16.1231b
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol - Plant 40:427–433. https://doi.org/10.1079/IVP2004541
Engelmann F (2014) Cryopreservation of clonal crops: a review of key parameters. Acta Hortic 1039:31–39. https://doi.org/10.17660/ActaHortic.2014.1039.2
Fira A, Clapa D, Badescu C (2008) Aspects regarding the in vitro propagation of highbush blueberry cultivar Blue crop. Bull UASVM Horticult 65:104–109
Giamperi F, Alvarez-Suarez JM, Battino M (2014) Strawberry and human health: effects beyond antioxidant activity. J Agr Food Chem 62:3867–3876. https://doi.org/10.1021/jf405455n
Gupta S, Tewari P (2020) Cryopreservation of Fragaria × ananassa using different techniques. Acta Hortic 1298:161–166. https://doi.org/10.17660/ActaHortic.2020.1298.23
Höfer M, Reed BM (2010) Cryopreservation of strawberry genetic resources in Germany. Acta Hort 918:139–146
Hunková J, Kleman J, Gažo J, Gajdošová A (2023) Adventitious regeneration of blackberry, blueberry, and kiwiberry and assessment of genetic stability by ISSR markers. Biologia 78:349–359. https://doi.org/10.1007/s11756-022-01211-7
Hunková J, Szabóová M, Gajdošová A (2021) Protocols for adventitious regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana.’ Plants 10:1155. https://doi.org/10.3390/plants10061155
Kami D, Kikuchi T, Sugiyama K, Suzuki T (2009) Cryopreservation of shoot apices of cranberry and highbush blueberry in-vitro cultures. Cryobiology 59:411–412. https://doi.org/10.1016/j.cryobiol.2009.10.162
Kim HH, Lee YG, Shin DJ, Ko HC, Gwag JG, Cho EG, Engelmann F (2009) Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 30:320–334
Le Bras C, Le Besnerais PH, Hamama L, Grapin A (2014) Cryopreservation of ex-vitro grown Rosa chinensis ‘Old Blush’ buds using droplet-vitrification and encapsulation-dehydration. Plant Cell Tiss Org 116:235–242. https://doi.org/10.1007/s11240-013-0400-5
Leunufna S, Keller ERJ (2021) In vitro multiplication rate and pot yield of cryopreservation derived buds of yams (Dioscorea spp). J Agri Res 6:000258. https://doi.org/10.23880/oajar-16000258
Matsumoto T (2017) Cryopreservation of plant genetic resources: conventional and new methods. Rev Agri Sci 5:13–20. https://doi.org/10.7831/ras5.13
Matsumoto T, Niino T (2014) The development of plant vitrification solution 2 and recent PVS2-based vitrification protocols. Acta Hortic 1039:21–28. https://doi.org/10.17660/ActaHortic.2014.1039.1
Matsumoto T, Yamamoto S, Fukui K, Niino T (2014) Cryopreservation of blueberry dormant shoot tips using V cryo-plate method. HortScience 49:S337–S338
Mazza G (2005) Compositional and functional properties of Saskatoon berry and blueberry. Int J Fruit Sci 5:101–120. https://doi.org/10.1300/J492v05n03_10
Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE (2002) Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J Agric Food Chem 50:519–525. https://doi.org/10.1021/jf011062r
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Niino T, Tanaka D, Ichikawa S, Tahano J, Ivette S, Shirata K, Uemura M (2003) Cryopreservation of in vitro – grown apical shoot tips of strawberry by vitrification. Plant Biotechnol 20:75–80
Niino T, Wunna Watanabe K, Nohara N, Rafique T, Yamamoto S, Fukui K, Arizaga M, Castillo Martinez CR, Matsumoto T, Engelmann F (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotechnol 31:281–287. https://doi.org/10.5511/plantbiotechnology.14.0624a
Niino T, Yamamoto S, Fukui K, Martínez CRC, Arizaga MV, Matsumoto T, Engelmann F (2013) Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) Basal stem buds on cryo-plates. CryoLetters 34:549–560
Niino T, Yamamoto S, Matsumoto T, Engelmann F, Valle Arizaga M, Tanaka D (2019) Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Hortic 1234:249–262. https://doi.org/10.17660/ActaHortic.2019.1234.33
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73. https://doi.org/10.1016/0168-9452(93)90189-7
Ochmian I, Kubus M, Dobrowolska A (2013) Description of plants and assessment of chemical properties of three species of Amelanchier genus. Dendrobiology 70:59–64. https://doi.org/10.12657/denbio.070.006
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55. https://doi.org/10.1016/j.plantsci.2004.07.022
Panis B, Thinh NT (2001) Cryopreservation of Musa germplasm. INIBAP Technical Guideline 5, International Network for the Improvement of Banana and Plantain, Bioversity International, Montpellier, France
Pawłowska B, Szewczyk-Taranek B (2014) Droplet vitrification cryopreservation of Rosa canina and Rosa rubiginosa using shoot tips from in situ plants. Sci Hortic-Amsterdam 168:151–156. https://doi.org/10.1016/j.scienta.2013.12.016
Pinker I, Halmagyi A, Olbricht K (2009) Effects of sucrose preculture on cryopreservation by droplet-vitrification of strawberry cultivars and morphological stability of cryopreserved plants. CryoLetters 30:202–211
Reed BM (2008) Cryopreservation of temperate berry crops. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer-Verlag, Berlin, pp 333–364
Reed BM, Hummer K (1995) Conservation of germplasm of strawberry (Fragaria species). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry: cryopreservation of plant germplasm I. Springer-Verlag, Berlin, pp 323–343
Reed BM, Schumacher L, Dumet D, Benson EE (2005) Evaluation of a modified encapsulation-dehydration procedure incorporating sucrose pretreatments for the cryopreservation of Ribes germplasm. In Vitro Cell Dev Biol - Plant 41:431–436. https://doi.org/10.1079/IVP2005664
Roque-Borda CA, Kulus D, Vacaro de Souza A, Kaviani B, Festozo Vicente E (2021) Cryopreservation of agronomic plant germplasm using vitrification-based methods: an overview of selected case studies. Int J Mol Sci 22:6157. https://doi.org/10.3390/ijms22116157
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33. https://doi.org/10.1016/0168-9452(91)90052-A
Takimoto Y, Maki S, Tanaka D, Yamamoto SI, Niino T, Matsumoto T (2020) Rapid evaluation of the genetic stability of rabbiteye blueberry plants regenerated from cryopreserved shoot tips by using long primer-RAPD analysis. J Jpn Soc Agri Technol Man 25:71–76
Uchendu EE, Reed BM (2009) Desiccation tolerance and cryopreservation of in vitro grown blueberry and cranberry shoot tips. Acta Hortic 810:567–574
Vujović T, Chatelet Ph, Ružić Đ, Engelmann F (2015b) Cryopreservation of Prunus sp. using aluminium cryo-plates. Sci Hortic-Amsterdam 195:173–182. https://doi.org/10.1016/j.scienta.2015.09.016
Vujović T, Ruzic DJ, Cerović R (2015a) Optimization of droplet vitrification protocol for cryopreservation of in vitro grown blackberry shoot tips. Acta Hortic 1099:595–601. https://doi.org/10.17660/ActaHortic.2015.1099.72
Vujović T, Ružić Đ, Vranić D, Marjanović T (2020) Cryopreservation in vitro of apple shoot tips following droplet-vitrification. Acta Hortic 1289:1–8. https://doi.org/10.17660/ActaHortic.2020.1289.1
Vujović T, Sylvestre I, Ružić Đ, Engelmann F (2011) Droplet-vitrification of apical shoot tips of Rubus fruticosus L. and Prunus cerasifera Ehrh. Sci Hortic (Amst) 130:222–228. https://doi.org/10.1016/j.scienta.2011.06.049
Wang L-Y, Li Y-D, Sun H-Y, Liu H-G, Tang X-D, Wang Q-C, Zhang Z-D (2017) An efficient droplet-vitrification cryopreservation for valuable blueberry germplasm. Sci Hortic 219:60–69. https://doi.org/10.1016/j.scienta.2017.03.007
Wisskirchen R, Haeupler H, Albers F (1998) Standardliste der Farn- und Blütenpflanzen Deutschlands. Ulmer, Stuttgart
Yamamoto S, Fukui K, Rafique T, Khan NI, Martinez CRC, Sekizawa K, Matsumoto T, Niino T (2012) Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminium cryo-plates. Plant Gen Res 10:14–19. https://doi.org/10.1017/S1479262111000906
Yamamoto S, Rafique T, Priyantha WS, Fuki K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 32:256–265
Yamamoto S, Wuna T, Rafique T, Arizaga M, Fukui K, Cruz Gutierrez E, Castillo Martinez CR, Watanabe K, Niino T (2015) The aluminium cryo-plate increases efficiency of cryopreservation protocols for potato shoot tips. Am J Potato Res 92:250–257. https://doi.org/10.1007/s12230-014-9425-5
Zamecnik J, Faltus M, Bilavcik A (2021) Vitrification solutions for plant cryopreservation: modification and properties. Plants 10:2623. https://doi.org/10.3390/plants10122623
Acknowledgements
This publication was supported by the Operational program Integrated Infrastructure within the project: Demand-driven research for the sustainable and innovative food, Drive4SIFood 313011V336, cofinanced by the European Regional Development Fund, and by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, Contract No. 451-03-47/2023-01/200215.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vujović, T., Anđelić, T., Marković, Z. et al. Cryopreservation of highbush blueberry, strawberry, and saskatoon using V and D cryo-plate methods and monitoring of multiplication ability of regenerated shoots. In Vitro Cell.Dev.Biol.-Plant 60, 85–97 (2024). https://doi.org/10.1007/s11627-023-10399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10399-5