Abstract
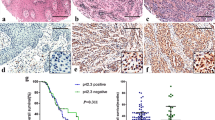
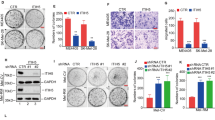
Solute carrier family 7 member 11 (SLC7A11)/xCT is an amino acid transporter that mediates the cystine uptake and glutamate export, participates in several malignant tumors’ progression. However, the role of SLC7A11 on the occurrence and development of melanoma still remains unclear. Here, the transcribed mRNA encoding for Cas9 and sgRNA targeting SLC7A11 in vitro were microinjected into zygotes, to establish the SLC7A11 knockout (KO) mice (SLC7A11−/−). Further, we conducted melanoma-bearing mice using the metastatic melanoma cell line (B16-F10) to observe the melanoma development. There was no off-target in KO mice detected by T7E1 cleavage assay. The results showed that the tumor volume of KO mice was significantly lower than that of SLC7A11+/+ (WT) mice at 8d, 10d, 12d, 14d, and 16d (P < 0.05). The tumors of WT appeared to more disorganized morphology, more unbalanced nuclear-cytoplasmic ratio, less defined boundary, and increased tumor necrosis. And after SLC7A11 deletion, the expression of CXCL9 and TLR6 were significantly up-regulated, and that of NOS2 and CCL8 were significantly down-regulated (P < 0.01). Additionally, Ki67 immunostaining revealed lower proliferating cells in the tumors of SLC7A11 KO mice compared to WT mice. In summary, the deletion of SLC7A11 significantly inhibited the development of melanoma. Our results provide direct evidence to identify SLC7A11 as a novel target for molecular therapy and prognosis judgment of melanoma.





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in https://doi.org/10.6084/m9.figshare.23513307.
References
Barbai T, Fejos Z, Puskas LG, Timar J, Raso E (2015) The importance of microenvironment: the role of CCL8 in metastasis formation of melanoma. Oncotarget 6:29111–29128
Chen Y, Hu SS, Mu L, Zhao BH, Wang MM, Yang NS, Bao GL, Zhu CG, Wu XS (2019) Slc7a11 modulated by POU2F1 is involved in pigmentation in rabbit. Int J Mol Sci 20:2493
Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT (2005) Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. P Natl Acad Sci USA 102:10964–10969
Ding Q, Lu PP, Xia YJ, Ding SP, Fan YH, Li X, Han P, Liu JM, Tian D, Liu M (2016) CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med-Us 5:3246–3259
Douguet L, Bod L, Lengagne R, Labarthe L, Kato M, Avril MF, Prevost-Blondel A (2016) Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of gamma delta 17 T cells in melanoma. Oncoimmunology 5:e1208878
Drayton RM, Dudziec E, Peter S, Bertz S, Hartmann A, Bryant HE, Catto JWF (2014) Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res 20:1990–2000
Galván I, Inácio Â, Dañino M, Corbí-Llopis R, Monserrat MT, Bernabeu-Wittel JJCE (2019) High SLC7A11 expression in normal skin of melanoma patients. Cancer Epidemiol 62:101582
Galván I, Moraleda V, Otero I, Álvarez E, Inácio  (2017) Genetic favouring of pheomelanin-based pigmentation limits physiological benefits of coloniality in lesser kestrels Falco naumanni. Mol Ecol 26:5594–5602
Guo WJ, Zhao YJ, Zhang ZF, Tan N, Zhao FY, Ge C, Liang LH, Jia DS, Chen TY, Yao M, Li JJ, He XH (2011) Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett 312:55–61
Jara J, Aroca P, Solano F, Martinez J, Lozano JA (1988) The role of sulfhydryl compounds in mammalian melanogenesis: the effect of cysteine and glutathione upon tyrosinase and the intermediates of the pathway. Biochim Biophys Acta 967:296–303
Ji XM, Qian J, Rahman SMJ, Siska PJ, Zou Y, Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, Rathmell JC, Young JD, Massion PP (2018) xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 37:5007–5019
Koppula P, Zhuang L, Gan BY (2021) Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12:599–620
Lai M, La Rocca V, Amato R, Freer G, Pistello M (2019) Sphingolipid/ceramide pathways and autophagy in the onset and progression of melanoma: novel therapeutic targets and opportunities. Int J Mol Sci 20:3436
Li C, Chen H, Lan Z, He S, Chen R, Wang F, Liu Z, Li K, Cheng L, Liu Y, Sun K, Wan X, Chen X, Peng H, Li L, Zhang Y, Jing Y, Huang M, Wang Y, Wang Y, Jiang J, Zha X, Chen L, Zhang H (2019) mTOR-dependent upregulation of xCT blocks melanin synthesis and promotes tumorigenesis. Cell Death Differ 26:2015–2028
Liu N, Zhang JL, Yin MZ, Liu H, Zhang X, Li JD, Yan B, Guo YY, Zhou JD, Tao J, Hu S, Chen X, Peng C (2021) Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol Ther 29:2321–2334
Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q (2011) MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. Febs Lett 585:1363–1367
Ma C-H, Jiang R, Li J-D, Wang B, Sun L-W, Lv Y (2014) Experimental study on residual tumor angiogenesis after cryoablation. Asian Pac J Cancer Prev 15:2491–2494
Ma MZ, Chen G, Wang P, Lu WH, Zhu CF, Song M, Yang J, Wen SJ, Xu RH, Hu YM, Huang P (2015) Xc(-) inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Lett 368:88–96
Mauldin IS, Wang E, Deacon DH, Olson WC, Bao YD, Slingluff CL (2015) TLR2/6 agonists and interferon-gamma induce human melanoma cells to produce CXCL10. Int J Cancer 137:1386–1396
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL (2022) Cancer treatment and survivorship statistics, 2022. Ca Cancer J Clin 72:409–436
Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, Robinson KC, Devi SP, Vanover JC, D’Orazio JA, McMahon M, Bosenberg MW, Haigis KM, Haber DA, Wang YS, Fisher DE (2012) An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 491:449–453
Moreno JA, Lambros MP (2015) The expression of SLC7A11 transporter in lung and pancreatic cancer tissues at different stages of development. Cancer Res 75:3209
Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, Reali UM, Giannotti B (1997) In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res 7:313–321
Motomura Y, Senju S, Nakatsura T, Matsuyoshi H, Hirata S, Monji M, Komori H, Fukuma D, Baba H, Nishimura Y (2006) Embryonic stem cell-derived dendritic cells expressing glypican-3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16–F10. Cancer Res 66:2414–2422
Pattanayak V, Lin S, Guilinger JP, Ma EB, Doudna JA, Liu DR (2013) High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31:839–843
Potterf SB, Virador V, Wakamatsu K, Furumura M, Santis C, Ito S, Hearing VJ (1999) Cysteine transport in melanosomes from murine melanocytes. Pigment Cell Res 12:4–12
Prados J, Melguizo C, Ortiz R, Boulaiz H, Carrillo E, Segura A, Rodriguez-Herva JJ, Ramos JL, Aranega A (2010) Regression of established subcutaneous B16–F10 murine melanoma tumors after gef gene therapy associated with the mitochondrial apoptotic pathway. Exp Dermatol 19:363–371
Prota G, Rorsman H, Rosengren AM, Rosengren E (1976) Phaeomelanic pigments from a human melanoma. Experientia 32:970–971
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308
Rodriguez-Cerdeira C, Gregorio MC, Lopez-Barcenas A, Sanchez-Blanco E, Sanchez-Blanco B, Fabbrocini G, Bardhi B, Sinani A, Guzman RA (2017) Advances in immunotherapy for melanoma: a comprehensive review. Mediat Inflamm 2017:1–14
Romano G, Paradiso F, Li P, Shukla P, Barger LN, El Naggar O, Miller JP, Liang RJ, Helms TL, Lazar AJ, Wargo JA, Taraballi F, Costello JC, Kwong LN (2023) Microparticle-Delivered Cxcl9 Prolongs Braf Inhibitor Efficacy in Melanoma. Cancer Immunol Res 11:558–569
Smalley KM, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA, Flaherty KT, Herlyn M (2007) Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Brit J Cancer 96:445–449
Tian YZ, Guo WN (2020) A review of the molecular pathways involved in resistance to BRAF inhibitors in patients with advanced-stage melanoma. Med Sci Monitor 26:e920957–e920951
Wang M, Cheng H, Wu H, Liu C, Li S, Li B, Su J, Luo S, Li Q (2022) Gambogenic acid antagonizes the expression and effects of long non-coding RNA NEAT1 and triggers autophagy and ferroptosis in melanoma. Biomed Pharmacother 154:113636
Yang NS, Mu L, Zhao BH, Wang MM, Hu SS, Zhao B, Chen Y, Wu XS (2018) RNAi-mediated SLC7A11 knockdown inhibits melanogenesis-related genes expression in rabbit skin fibroblasts. J Genet 97:463–468
Zhang L, Huang Y, Ling JJ, Zhuo WL, Yu Z, Luo YB, Zhu Y (2018) Overexpression of SLC7A11: a novel oncogene and an indicator of unfavorable prognosis for liver carcinoma. Future Oncol 14:927–936
Zhang Z, Kim K, Li X, Moreno M, Sharp T, Goodheart MJ, Safe S, Dupuy AJ, Amendt BA (2014) MicroRNA-26b represses colon cancer cell proliferation by inhibiting lymphoid enhancer factor 1 expression. Mol Cancer Ther 13:1942–1951
Acknowledgements
The research was financially supported by the National Natural Science Foundation of China (Grant no. 32172722), and Yangzhou University Student Innovation and Entrepreneurship Training Program Project (Grant no. X20220652) and Qing Lan Project of Yangzhou university.
Author information
Authors and Affiliations
Contributions
Yang Chen: Data curation, Formal analysis, Methodology, Writing—review & editing. Tingting Lu, Yufei Liu, Yongqi Liu: Data curation, Formal analysis, Methodology. Shaocheng Bai, Qiuran Chen, Bohao Zhao: Formal analysis, Software, Validation. Xinsheng Wu: Data curation, Funding acquisition, Supervision. All authors contributed to, edited, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval
The animal experiments were approved by the Animal Ethics Committee of Yangzhou University, China (approval No. 201802126) and were strictly implemented according to the Guide for the Care and Use of Laboratory Animals. The mice were sacrificed by cervical dislocation following anesthesia with Shutai.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Lu, T., Liu, Y. et al. Establishment of SLC7A11‐knockout mouse and its preliminary investigation in melanoma. In Vitro Cell.Dev.Biol.-Animal 59, 729–737 (2023). https://doi.org/10.1007/s11626-023-00819-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00819-6