Abstract
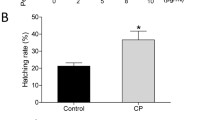
The major objective of this study was to improve the development rate of parthenogenetic porcine embryos. In this study, the anti-oxidative and anti-apoptotic effects of three antioxidants, β-mercaptoethanol (β-ME), α-tocopherol, and extracellular superoxide dismutase (EC-SOD), were examined on the development of parthenogenetic porcine embryos. The development rate of parthenogenetic porcine embryos to the blastocyst stage was 8.1% for control; 19.1%, 14.6%, and 5.0% for 1, 3, and 5 μM β-ME; 17.2% and 17.5% for 50 and 100 μM α-tocopherol and 12.0% and 4.0% for EC-SOD transgenic mouse embryonic fibroblast (Tg-MEF) and EC-SOD non-transgenic mouse embryonic fibroblast (NTg-MEF) conditioned medium at day 3, respectively. Here, β-ME, α-tocopherol, and EC-SOD Tg-MEF conditioned medium increased the development rate of parthenogenetic porcine embryos to the blastocyst stage (P < 0.05). The average number of total cells and apoptotic cells at the blastocyst was analyzed at the optimal conditions of the three antioxidants. The three antioxidants increased the average number of total cells at the blastocyst, and they decreased apoptotic cells at the blastocyst as compared to control without supplementation (P < 0.05). When the reactive oxygen species levels in two-cell embryos after 1 μM β-ME and 100 μM α-tocopherol treatment were examined, those were lower than control group (P < 0.05). In conclusion, it was found that the three antioxidants, β-mercaptoethanol, α-tocopherol, and EC-SOD Tg-MEF, conditioned medium can play a role as a strong stimulator in the development of parthenogenetic porcine embryos.




Similar content being viewed by others
References
Abeydeera L. R.; Wang W. H.; Cantley T. C.; Prather R. S.; Day B. N. Presence of beta-mercaptoethanol can increase the glutathione content of pig oocytes matured in vitro and the rate of blastocyst development after in vitro fertilization. Theriogenology 50: 747–756; 1998.
Aitken R. J.; Clarkson J. S.; Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 41: 183–197; 1989.
Caamano J. N.; Ryoo Z. Y.; Thomas J. A.; Youngs C. R. beta-mercaptoethanol enhances blastocyst formation rate of bovine in vitro-matured/in vitro-fertilized embryos. Biol. Reprod. 55: 1179–1184; 1996.
Che L.; Lalonde A.; Bordignon V. Chemical activation of parthenogenetic and nuclear transfer porcine oocytes using ionomycin and strontium chloride. Theriogenology 67: 1297–1304; 2007.
Choi J.; Park S. M.; Lee E.; Kim J. H.; Jeong Y. I.; Lee J. Y.; Park S. W.; Kim H. S.; Hossein M. S.; Jeong Y. W.; Kim S.; Hyun S. H.; Hwang W. S. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 75: 1127–1135; 2008.
Cui X. S.; Kim N. H. Polyamines inhibit apoptosis in porcine parthenotes developing in vitro. Mol. Reprod. Dev. 70: 471–477; 2005.
de Matos D. G.; Furnus C. C.; Moses D. F.; Martinez A. G.; Matkovic M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Mol. Reprod. Dev. 45: 451–457; 1996.
Dennery P. A. Effects of oxidative stress on embryonic development. Birth Defects Res. C. Embryo Today 81: 155–162; 2007.
Freeman B. A.; Crapo J. D. Biology of disease: free radicals and tissue injury. Lab. Invest. 47: 412–426; 1982.
Funahashi H. Effect of beta-mercaptoethanol during in vitro fertilization procedures on sperm penetration into porcine oocytes and the early development in vitro. Reproduction 130: 889–898; 2005.
Goto Y.; Noda Y.; Narimoto K.; Umaoka Y.; Mori T. Oxidative stress on mouse embryo development in vitro. Free Radic. Biol. Med. 13: 47–53; 1992.
Guerin P.; El Mouatassim S.; Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Updat. 7: 175–189; 2001.
Halliwell B.; Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 281: 9–19; 1991.
Hossein M. S.; Hashem M. A.; Jeong Y. W.; Lee M. S.; Kim S.; Kim J. H.; Koo O. J.; Park S. M.; Lee E. G.; Park S. W.; Kang S. K.; Lee B. C.; Hwang W. S. Temporal effects of alpha-tocopherol and L-ascorbic acid on in vitro fertilized porcine embryo development. Anim. Reprod. Sci. 100: 107–117; 2007.
Jeong Y. W.; Park S. W.; Hossein M. S.; Kim S.; Kim J. H.; Lee S. H.; Kang S. K.; Lee B. C.; Hwang W. S. Antiapoptotic and embryotrophic effects of alpha-tocopherol and L-ascorbic acid on porcine embryos derived from in vitro fertilization and somatic cell nuclear transfer. Theriogenology 66: 2104–2112; 2006.
Kim K.; Lerou P.; Yabuuchi A.; Lengerke C.; Ng K.; West J.; Kirby A.; Daly M. J.; Daley G. Q. Histocompatible embryonic stem cells by parthenogenesis. Science 315: 482–486; 2007.
Kim S. H.; Kim M. O.; Gao P.; Youm C. A.; Park H. R.; Lee T. S.; Kim K. S.; Suh J. G.; Lee H. T.; Park B. J.; Ryoo Z. Y.; Lee T. H. Overexpression of extracellular superoxide dismutase (EC-SOD) in mouse skin plays a protective role in DMBA/TPA-induced tumor formation. Oncol. Res. 15: 333–341; 2005.
Kitagawa Y.; Suzuki K.; Yoneda A.; Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 62: 1186–1197; 2004.
Lee S. R.; Kim M. O.; Kim S. H.; Kim B. S.; Yoo D. H.; Park Y. S.; Park Y. B.; Ha J. H.; Ryoo Z. Y. Effect of conditioned medium of mouse embryonic fibroblasts produced from EC-SOD transgenic mice in nuclear maturation of canine oocytes in vitro. Anim. Reprod. Sci. 99: 106–116; 2007.
Li J.; Foote R. H. Culture of rabbit zygotes into blastocysts in protein-free medium with one to twenty per cent oxygen. J. Reprod. Fertil. 98: 163–167; 1993.
Lim J. M.; Liou S. S.; Hansel W. Intracytoplasmic glutathione concentration and the role of beta-mercaptoethanol in preimplantation development of bovine embryos. Theriogenology 46: 429–439; 1996.
Marnett L. J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 424: 83–95; 1999.
McEvoy T. G.; Coull G. D.; Broadbent P. J.; Hutchinson J. S.; Speake B. K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod. Fertil. 118: 163–170; 2000.
Meister A. Selective modification of glutathione metabolism. Science 220: 472–477; 1983.
Orsi N. M.; Leese H. J. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol. Reprod. Dev. 59: 44–53; 2001.
Sturmey R. G.; Leese H. J. Energy metabolism in pig oocytes and early embryos. Reproduction 126: 197–204; 2003.
Takahashi M.; Nagai T.; Hamano S.; Kuwayama M.; Okamura N.; Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol. Reprod. 49: 228–232; 1993.
Yang H. W.; Hwang K. J.; Kwon H. C.; Kim H. S.; Choi K. W.; Oh K. S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 13: 998–1002; 1998.
Yi Y. J.; Park C. S. Parthenogenetic development of porcine oocytes treated by ethanol, cycloheximide, cytochalasin B and 6-dimethylaminopurine. Anim. Reprod. Sci. 86: 297–304; 2005.
Acknowledgments
This work was supported in part by Grant-in Aid from Rural Development Administration, Technology Development Program for Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries, Republic of Korea and Grant of the Korean Ministry of Education, Science, and Technology (The Regional Core Research Program).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: J. Denry Sato.
Rights and permissions
About this article
Cite this article
Yuh, H.S., Yu, D.H., Shin, M.J. et al. The effects of various antioxidants on the development of parthenogenetic porcine embryos. In Vitro Cell.Dev.Biol.-Animal 46, 148–154 (2010). https://doi.org/10.1007/s11626-009-9250-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-009-9250-1