Abstract
Background
Obesity is associated with elevated blood pressure (BP). In patients with obesity and hypertension, weight loss lowers BP, but the long-term effect of weight loss on BP is less clear.
Objective
We aimed to assess the effect of long-term weight loss intervention on BP in normotensive and hypertensive subjects.
Design
Randomized controlled trial.
Participants
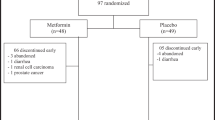
Two hundred seventy-eight subjects (mean age 47.9 ± 9.3 years, 89% male, 56% hypertensive) with abdominal obesity or elevated serum triglycerides and low high-density lipoprotein cholesterol were recruited.
Intervention
Eighteen-month weight loss intervention.
Main Measures
Body weight and BP were measured at baseline, after 6 and 18 months.
Results
After 6 months of intervention, in the weight loss phase, body mass index (BMI) decreased by an average of −2.2±1.5 kg/m2 (p<0.001) and both diastolic BP (DBP) and systolic BP (SBP) decreased by −2.1±8.8 mmHg and −2.3±12.9 mmHg, respectively (p<0.01 for both). The change in BMI was similar in normotensive and hypertensive subjects (−2.0±1.6 and −2.3±1.5, p = 0.246). However, DBP and SBP decreased significantly (−5.2±7.1 mmHg and −6.2±12.5 mmHg, respectively, p<0.001 for both) in hypertensive subjects, and increased in normotensive subjects (1.8±9.3 mmHg, p = 0.041 and 2.7±11.7 mmHg, p = 0.017, respectively). After 18 months, in the weight maintenance phase, BMI slightly increased (0.9±1.3 kg/m2, p<0.001) but remained significantly lower than at baseline (p<0.0001). Unlike BMI, DBP and SBP increased significantly in hypertensive subjects (p<0.001) and returned almost to baseline levels.
Conclusion
Weight-loss intervention reduced BP in hypertensive patients, but this was not maintained in the long run.
Clinical Trial Registration
ClinicalTrials.gov Identifier: NCT01530724
Similar content being viewed by others

INTRODUCTION
Obesity is a worldwide epidemic, with estimated prevalence of 39% for overweight (body mass index (BMI) between 25 and 30) and 13% for obese (BMI>30) among adults (aged ≥18 years).1, 2 Obesity is associated with elevated blood pressure (BP), which comprises a major risk factor for cardiovascular disease.3, 4 Hypertension, defined as systolic blood pressure (SBP) above 130 mmHg or diastolic blood pressure (DBP) above 80 mmHg5, appears in 60 to 77% of people with obesity.6 According to the Framingham Heart Study, 65% of the risk for hypertension in women and 78% in men is related to increased adiposity.7 Thus, lifestyle interventions to achieve weight loss, such as diet and exercise, are recommended for high BP management.5, 8, 9 Weight loss interventions can be divided into a weight loss phase, which usually lasts 4–6 months, followed by a weight maintenance phase.10 During the weight maintenance phase, body weight either stays stable, increases slightly, or is fully regained, despite adherence to the diet, due to physiological adaptations (reducing metabolic rate and increasing appetite) and persisting obesogenic environments that contribute to weight regain.11 The effect of short-term weight loss on BP is very robust. In a meta-analysis that summarized 25 randomized controlled studies, which included 50% hypertensive subjects from different populations and with various BMIs, weight loss of ~5 kg was associated with an average BP reduction of 4.4 mmHg and 3.6 mmHg for SBP and DBP, respectively.12 Thus, it was suggested that weight loss greatly contributes to hypertension treatment. On the other hand, long-term studies on the relationship between weight loss and BP reduction are inconclusive.13,14,15,16 A systematic review found that long-term (≥2 years) dietary interventions for weight loss were effective in reducing BP, and that long-term weight change (either reduced, maintained, or elevated) accounts for 30% of SBP differences.13 Contrary to this review, a 26 weeks intervention study found that during weight maintenance phase, BP increased significantly, despite stable weight.14 In this analysis of the CENTRAL trial17, 18, we aimed to evaluate the BP changes during the weight loss and the weight maintenance phases of the weight loss intervention and to assess whether the effect of weight loss on BP is the same among normotensive and hypertensive subjects.
METHODS
This is a secondary analysis of the 18 months long CENTRAL trial (ClinicalTrials.gov Identifier: NCT0153072419, 20), which was conducted between October 2012 and April 2014 in a research center workplace in Dimona, Israel. Inclusion criteria were abdominal obesity (waist circumference (WC)>102 cm (40 inches) for men and >88 cm (35 inches) for women), or serum triglycerides (TG)>150 mg/dL and high-density lipoprotein cholesterol (HDL-c)<40 mg/dL for men and <50 mg/dL for women. Exclusion criteria were serum creatinine≥2 mg/dL, impaired liver function (≥3 fold the upper level of ALT and AST enzymes), active cancer, pregnant or lactating women, being highly physically active (more than 3 h/week) or inability to initiate physical activity, or participation in another trial. Participants that met the study criteria were recruited by the on-site medical clinic via personal meeting. The study protocol was approved by the Soroka University Medical Center Ethics Board and Helsinki Committee. All participants provided written informed consent and did not receive any financial compensation.
Intervention
After completion of the baseline measurements, participants were instructed to consume low-fat diet (LF, n = 139) or low-carbohydrate/Mediterranean diet (LC/MED, n = 139) for the entire period of the study (18 months), based on the assigned group.21 After six months of weight loss intervention, participants in each group were further randomized into “with-” or “without moderate physical activity” groups. The participants were aware of the intervention to which they were assigned (open-label). Study investigators assessing outcomes were blinded to the group assignments.
Diet Intervention
Both diets aimed for a moderate, long-term, weight loss at an energy intake of 1500 kcal/day for women and 1800 kcal/day for men, restricted intake of trans-fats, refined carbohydrates, and increased vegetables. Lunch, typically the main meal in this population, was adjusted to the specific diet groups and was provided by the workplace cafeteria. The 18-month diet intervention included a 90-min nutritional session in the workplace with clinical dietitians every week during the first month of intervention, and once a month thereafter. To maintain equal intensity of treatment for both diets, the workshop format and the quality of the materials were similar across the diet groups, except for instructions and materials specific to each diet strategy.18 Further details may be found in our original publication.18
For the LF diet, the aim was to limit total fat intake to 30% of calories, with up to 10% of saturated fat, and no more than 300 mg of cholesterol per day, and to increase dietary fiber. Participants were counseled to consume whole grains, vegetables, fruits, and legumes and to limit their consumption of additional fats, sweets, and high-fat snacks.
The MED/LC diet combined the Mediterranean and low-carbohydrate diets described in our previous weight loss trial.18 The diet restricted carbohydrate intake to less than 40 g/day in the first two months (induction phase), and thereafter a gradual increase up to 70 g/day, and increased protein and fat intake, according to the MED diet. The MED/LC diet was rich in vegetables and legumes and low in red meat, with poultry and fish replacing beef and lamb. This group was also provided 28 g of walnuts/day (160 kcal/84% fat, mostly PUFA (omega-3 α-linolenic acid)) starting from the third month.
Clinical Measurements
Clinical parameters were measured at baseline and after 6 and 18 months of weight loss intervention. Body weight was measured monthly without shoes to the nearest 0.1 kg. Height was measured to the nearest millimeter using a standard wall-mounted stadiometer. Waist circumference was measured half-way between the last rib and the iliac crest to the nearest millimeter using a standard procedure using a 150-cm anthropometric measuring tape. Fasting blood samples were taken at 8 a.m. and were stored at −80 °C. All biochemical analyses were performed at Leipzig laboratories, Germany.
Participants were asked to avoid coffee or alcohol consumption 12 h prior to BP measurements. Blood pressure was measured twice, 1 min apart, in sitting position, in a quiet room following 5 min of rest, using an automatic BP monitor (Datascope Accutorr 4 [Datascope]). Cuff size was adjusted to fit the arm circumference. During the intervention period, cuff size was re-adjusted due to changes in arm circumference after weight loss/regain. The second measurement was taken for our calculations.
Statistical Analysis
The primary outcome in this report was change in BP over 18 months of weight-loss intervention, with a priori hypothesis that BP changes will parallel to changes in body mass index. Results are presented as mean ± standard deviation unless otherwise stated. Independent t test was used to compare between diet groups at baseline. Paired samples t test was used to test for significant changes of BMI and BP values at 6 and 18 months compared with baseline for the entire study population and stratified for normotensive and hypertensive (DBP > 80, SBP > 130 mmHg, or medication use)5 groups. Linear regression was used to evaluate the relationships between DBP and SBP to body weight. Linear regression analyses was also used to test the trend between quartiles of changes in BMI and changes in DBP and SBP. Sample size calculations were based on results of a previous 12 months study among 34 morbidly obese patients following bariatric surgery22, considering an alpha = 5% and power = 80%, with the conservative assumption of a mean detectable SBP difference of 19 mmHg between the time intervals. We used normotensive vs. hypertensive for power calculation, as conservative estimation, by using a clinical group expected to be less responsive to our cohort. Statistical analysis was performed using IBM SPSS Version 20.0 (SPSS, Inc., Chicago, IL, USA). All tests were two-tailed and p<0.05 was considered statistically significant.
RESULTS
Subjects
Two hundred and seventy-eight subjects (male − 247) with a mean age of 47.9 years (range 28–70 years) and mean BMI of 30.9 kg/m2 (range 24–44 kg/m2) volunteered to participate in this study. One hundred and fifty-six participants (56.1%) met the American Heart Association (AHA) criteria for hypertension, among them, 23 (8.3%) used anti-hypertensive medication.5 Baseline characteristics of the study population are given in Table 1. Hypertensive participants were older and had higher BMI, waist circumference, and glycemic profile as compared to normotensive (p<0.05 for all). There were no significant differences in baseline lipid profile between intervention groups (p>0.093).
The Association Between Body Weight and Blood Pressure
In the entire group, baseline BMI was correlated with both DBP and SBP (r = 0.211, p<0.001; r = 0.205, p = 0.001, respectively). After 6 months of weight loss intervention, at the end of the weight-loss phase, BMI decreased by an average of −2.2 ± 1.5 kg/m2 (Fig. 1, p<0.001). Similarly, both DBP and SBP were significantly reduced after 6 months compared to baseline (−2.1 ± 8.8 mmHg, p<0.001; −2.3 ± 12.9 mmHg, p = 0.006, respectively). After 18 months of intervention, BMI slightly increased (0.9 ± 1.3 kg/m2), but remained significantly lower than at baseline (p<0.001) (Fig. 1). Unlike BMI, both DBP and SBP increased significantly (p<0.001) during the weight maintenance phase, and returned nearly to baseline levels. No significant change in hypertensive medication was achieved. After 6 months, the change in BMI was associated with a decrease in both DBP and SBP (r = 0.163, p = 0.010; r = 0.248, p<0.001 respectively). These associations remained significant also after 18 months, at the end of the weight maintenance phase (r = 0.145, p = 0.029; r = 0.163, p = 0.014 respectively). When stratified by gender, similar trends were achieved in both males and females.
Changes in body mass index and diastolic and systolic blood pressure during weight loss (6 months) and weight maintenance phases (18 months) of dietary intervention. Black line, BMI (body mass index); orange line, DBP (diastolic blood pressure); blue line, SBP (systolic blood pressure). Paired samples t test was used for between time-points comparison. (a) Significantly different from time 0. (b) Eighteen months significantly different from 6 months. Statistical significant—p<0.05.
The Effect of Weight Loss on Blood Pressure in Normotensive and Hypertensive Subjects
The change in body weight, after 6 months and after 18 months, was similar in normotensive and hypertensive subjects (Fig. 2A). However, unlike the change in BMI, the changes in BP between the two groups showed different trends. After 6 months of intervention, DBP and SBP decreased significantly (−5.2 ± 7.1 mmHg and −6.2 ± 12.5 mmHg, respectively, p<0.001 for both) in the hypertensive group, whereas DBP and SBP increased significantly (1.8 ± 9.3 mmHg, p = 0.041 and 2.7 ± 11.7 mmHg, p = 0.017 respectively) in the normotensive group (Fig. 2A, B). After 18 months, the normotensive group showed an additional increase in DBP (2.3 ± 8.2) (p<0.001 vs. baseline and p = 0.006 vs. 6 months), and a slight increase in SBP (2.2 ± 11.6) (p< 0.001 vs. baseline and p = ns vs. 6 months). In the hypertensive group, DBP slightly increased (2.0 ± 8.0) but remained significantly lower than at baseline (p<0.001) while SBP increased by 4.3 ± 14.7 and was no longer significantly different from baseline (p = 0.096).
Changes in body mass index and blood pressure during the intervention among normotensive and hypertensive participants. Changes in (A) body mass index and (B) diastolic and (C) systolic blood pressure among normotensive (green line) and hypertensive (red line) participants, during weight loss (6 months) and weight maintenance (18 months) phases of dietary intervention. Paired samples t test was used for between time-points comparison. (a) Significantly different from time 0. (b) Eighteen months significantly different from 6 months. Independent t test was used for between groups comparison. Statistical significant—p<0.05.
After 6 months, the changes in DBP were not associated with the changes in BMI in the normotensive group (p of trend = 0.349) (Fig. 3A), while in the hypertensive group the decrease in BMI showed a trend towards reduction in DBP (p of trend = 0.058) (Fig. 3A). The decrease in BMI in both groups was associated with the decrease in SBP (p of trend = 0.011 in the hypertensive group and p of trend = 0.002 in the normotensive group) (Fig. 3B). Among hypertensive subjects, 18 months reduction in BMI was associated with changes in DBP only (p of trend = 0.005) (Fig. 3C, D).
The relation between changes in body mass index and blood pressure among hypertensive and normotensive groups. Mean change in (A, C) diastolic and (B, D) systolic blood pressure after 6 and 18 months of dietary intervention between quartiles of BMI changes, among normotensive (black bars) and hypertensive (gray bars) groups. Paired samples t test was used for between time-points comparison. Linear regression analysis was used to assess the trend between BMI change quartiles and changes in blood pressure. *p<0.05.
DISCUSSION
In the present study, we show that weight loss is associated with short-term decrease in BP among hypertensive, but not among normotensive subjects. However, this effect is not maintained in the long run despite sustained weight loss.
It is well established that short-term caloric restriction and weight loss lead to BP decrease through suppression of the sympathetic nervous system and the renin-angiotensin system19, 20. The effect of weight loss on BP in normotensive subjects is less well investigated. Several studies included hypertensive and normotensive subjects, but they did not analyze the data separately for normotensive and hypertensive sujects.21 In most studies where normotensive subjects were included, they were defined according to the traditional definition (less than 140/90 mmHg). However, according to the current AHA definition, values above 130/80 are considered as hypertension, and therefore we used the new cut point for definition of normotensive subjects (less than 130/80 mmHg).5 Most studies evaluated the effect of surgery-induced weight reduction22,23,24,25,26,27 and included normotensive subjects. During recent years, it has been shown that weight loss after bariatric surgery is associated with a remarkable BP decrease.28,29,30,31,32,33,34 In some studies, the reduction in BP post-surgery was observed in both hypertensive and normotensive subjects.35, 36 However, the effect of surgery may be mediated not only via weight reduction, as the decrease in BP is observed shortly post-surgery, even before a significant weight reduction is achieved, suggesting involvement of neural and hormonal mechanisms in the BP reduction.37, 38 Unlike studies that evaluated the effect of bariatric surgery on BP and included hypertensive and normotensive subjects, most studies that evaluated the effect of dietary intervention on BP included only hypertensive subjects.39 In our study, evaluating the effect of weight loss intervention, we included both normotensive and hypertensive subjects. The weight loss was modest, thus might explain why we did not observe BP decrease in normotensive subjects. We also used the more strict criteria for definition of hypertension, and we believe that some normotensives in other studies could have been defined as hypertensives in our study. Flores et al. showed that bariatric surgery can lower BP in hypertensive and normotensive subjects, but the BP decrease was mild in normotensive subjects and they used a cut point of less than 140/90 mmHg to define normotensive subjects.27 Thus, our normotensive subjects had low initial BP, and in these subject’s weight reduction was not associated with BP reduction and may even be associated with BP increase. It is noteworthy that even after bariatric surgery some authors did not observe BP reduction in normotensive subjects. Bonfils PK. et al. evaluated 24-h ambulatory blood pressure monitoring the effect of gastric bypass on BP in hypertensive and normotensive subjects.23 Six weeks after surgery, body weight decreased similarly in both groups; however, BP decreased only in hypertensive subjects. Dall’Asta, C. et al. evaluated the effect of weight loss on BP, in hypertensive and normotensive subjects, one year after gastric bypass.26 They showed a significant decrease in BP and in plasma renin activity and aldosterone levels only in the hypertensive patients. These findings may suggest that when the renin-angiotensin system is not activated, as in normotensive subjects, weight reduction has no effect on BP. It is noteworthy that in normotensive subjects weight loss was associated with even a slight increase in BP. In obese patients, hypertension is attributed in part to over activity of the sympathetic nervous system. In obese normotensive subjects, the sympathetic nervous system is probably not hyperactive. In these subjects, maintaining a low caloric diet is a stressful condition that activates the sympathetic nervous system and may exceed the expected suppression from weight loss. Thus, the overall effect of weight loss in normotensive subjects is activation of the sympathetic nervous system and BP increase.
It is clear that in hypertensive patients, weight reduction is associated with BP decrease. The main question is whether it can be maintained in the long run. In the present study, we show that the effect of weight loss on BP is not maintained over time even when the weight is still lower than baseline. The effect of diet and refeeding was assessed in animal models. In obese rats undergoing a supplemented fast, BP fell almost immediately but then stabilized despite continuing weight loss.
The depressor effect of fasting was reversed within 2 days of refeeding.40 These findings suggest that nutritional state, but not body weight, has important effects on BP. Similar observations were described in humans. In postmenopausal women, even partial weight regain following intentional weight loss was associated with increased cardiometabolic risk.41 The effects of minimal weight regain after weight loss have been reported in 2 studies.42, 43 Wang et al.43 demonstrated decreases in systolic and diastolic BP (8 and 11%, respectively) with weight loss, but these values returned close to baseline with 2% weight regain. Linna et al.42 observed decreases in diastolic BP (9%) with weight loss, while systolic values remained unaffected. During the weight maintenance phase, systolic values rose by 3% and diastolic values returned to baseline with 2% weight regain. Thus, even small amounts of weight regain (2%) may completely reverse the beneficial effects of weight loss on BP.42, 43 Since most patients regain weight in the long run, maintaining long-term BP decrease may be impossible. In the Swedish Obese Subjects (SOS) study, the incidence rates of hypertension did not differ between the surgically treated group and the control group after 2 and 10 years.44 This finding also suggests that the BP decrease achieved with weight loss usually cannot be maintained in the long run.
Our study has some limitations. The BP levels were not monitored by 24-h ambulatory blood pressure recording. However, the measurements were performed meticulously using an automatic BP monitor, and most previous studies did not use 24-h ambulatory blood pressure monitoring either. We have only 2 points of evaluation—after 6 and after 18 months—nevertheless, we were able to show the difference in BP levels after 6 and 18 months. Indeed some of the BP changes were mild but even a mild change in BP has an impact on cardiovascular morbidity and mortality in the general population. The patients used different diets to lose weight, but our scope was the association between weight loss and BP and therefore the diet was less relevant. In addition, due to the sex distribution of the workplace in which this study was conducted, the results may be less applied to women (11% of the study participants). However, in a sensitive analysis, similar trends were found between men and women.
In conclusion, our study shows that weight-loss intervention may reduce BP in hypertensive participants, but this is not maintained in the long run.
References
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367-385.
Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51-60.
Lanas F, Seron P. Diverging trends in obesity, diabetes, and raised blood pressure in the Americas. Lancet Glob Health. 2020;8:e18-e19.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904-10.
Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067-74.
Fantin F, Giani A, Zoico E, Rossi AP, Mazzali G, Zamboni M. Weight Loss and Hypertension in Obese Subjects. Nutrients. 2019;11.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021-3104.
Bluher M, Rudich A, Kloting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35:342-9.
Lean M, Hankey C. Keeping it off: the challenge of weight-loss maintenance. Lancet Diabetes Endocrinol. 2018;6:681-683.
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878-84.
Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension. 2009;54:756-62.
Engberink MF, Geleijnse JM, Bakker SJ, et al. Effect of a high-protein diet on maintenance of blood pressure levels achieved after initial weight loss: the DiOGenes randomized study. J Hum Hypertens. 2015;29:58-63.
Fazliana M, Liyana AZ, Omar A, et al. Effects of weight loss intervention on body composition and blood pressure among overweight and obese women: findings from the MyBFF@home study. BMC Womens Health. 2018;18:93.
Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1-11.
Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71:379-388.
Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of Distinct Lifestyle Interventions on Mobilization of Fat Storage Pools: CENTRAL Magnetic Resonance Imaging Randomized Controlled Trial. Circulation. 2018;137:1143-1157.
Grossman E, Eshkol A, Rosenthal T. Diet and weight loss: their effect on norepinephrine renin and aldosterone levels. Int J Obes. 1985;9:107-14.
Sowers JR, Nyby M, Stern N, et al. Blood pressure and hormone changes associated with weight reduction in the obese. Hypertension. 1982;4:686-91.
Noren E, Forssell H. Very low calorie diet without aspartame in obese subjects: improved metabolic control after 4 weeks treatment. Nutr J. 2014;13:77.
Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9:559-68.
Bonfils PK, Taskiran M, Damgaard M, et al. Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase in mid-regional pro-atrial natriuretic peptide in morbid obese patients. J Hypertens. 2015;33:1215-25.
Busetto L, De Stefano F, Pigozzo S, Segato G, De Luca M, Favretti F. Long-term cardiovascular risk and coronary events in morbidly obese patients treated with laparoscopic gastric banding. Surg Obes Relat Dis. 2014;10:112-20.
Careaga M, Esmatjes E, Nunez I, Molero J, Vidal J, Flores L. Effect of weight loss on abnormal 24-hour blood pressure patterns in severely obese patients. Surg Obes Relat Dis. 2016;12:1719-1724.
Dall’Asta C, Vedani P, Manunta P, et al. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19:110-4.
Flores L, Vidal J, Canivell S, Delgado S, Lacy A, Esmatjes E. Hypertension remission 1 year after bariatric surgery: predictive factors. Surg Obes Relat Dis. 2014;10:661-5.
Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of Bariatric Surgery in Obese Patients With Hypertension: The GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation. 2018;137:1132-1142.
Heneghan HM, Meron-Eldar S, Brethauer SA, Schauer PR, Young JB. Effect of bariatric surgery on cardiovascular risk profile. Am J Cardiol. 2011;108:1499-507.
Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25:397-405.
Ricci C, Gaeta M, Rausa E, Macchitella Y, Bonavina L. Early impact of bariatric surgery on type II diabetes, hypertension, and hyperlipidemia: a systematic review, meta-analysis and meta-regression on 6,587 patients. Obes Surg. 2014;24:522-8.
Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763-77.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292:1724-37.
Wilhelm SM, Young J, Kale-Pradhan PB. Effect of bariatric surgery on hypertension: a meta-analysis. Ann Pharmacother. 2014;48:674-82.
Pedersen JS, Borup C, Damgaard M, et al. Early 24-hour blood pressure response to Roux-en-Y gastric bypass in obese patients. Scand J Clin Lab Invest. 2017;77:53-59.
Hawkins DN, Faler BJ, Choi YU, Prasad BM. Time Course of Blood Pressure Decrease After Bariatric Surgery in Normotensive and Hypertensive Patients. Obes Surg. 2018;28:1845-1851.
Yin X, Qian J, Wang Y, et al. Short-term outcome and early effect on blood pressure of laparoscopic sleeve gastrectomy in morbidly obese patients. Clin Exp Hypertens. 2019;41:622-626.
Pouwels S, Lascaris B, Nienhuijs SW, Bouwman AR, Buise MP. Short-Term Changes in Cardiovascular Hemodynamics in Response to Bariatric Surgery and Weight Loss Using the Nexfin(R) Non-invasive Continuous Monitoring Device: a Pilot Study. Obes Surg. 2017;27:1835-1841.
Gilardini L, Redaelli G, Croci M, Conti A, Pasqualinotto L, Invitti C. Effect of a Modest Weight Loss in Normalizing Blood Pressure in Obese Subjects on Antihypertensive Drugs. Obes Facts. 2016;9:251-8.
Ernsberger P, Nelson DO. Refeeding hypertension in dietary obesity. Am J Physiol. 1988;254:R47-55.
Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2013;68:691-8.
Linna MS, Borg P, Kukkonen-Harjula K, et al. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int J Obes (Lond). 2007;31:245-53.
Wang P, Holst C, Wodzig WK, et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int J Obes (Lond). 2012;36:1545-51.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-93.
Acknowledgements
The authors thank the CENTRAL participants for their consistent cooperation and to Iris Shai from Ben-Gurion University of Negev.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors have no relevant conflict of interest to disclose and have approved the final article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gepner, Y., Goldstein, N., Shelef, I. et al. Dissociation Between Long-term Weight Loss Intervention and Blood Pressure: an 18-month Randomized Controlled Trial. J GEN INTERN MED 36, 2300–2306 (2021). https://doi.org/10.1007/s11606-021-06655-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06655-2