Abstract
Purpose
The dysregulation of brain iron homeostasis is closely relevant to a multitude of chronic neurological disorders. This study employed quantitative susceptibility mapping (QSM) to detect and compare whole-brain iron content between childhood epilepsy with centrotemporal spikes (CECTS) children and typically developing children.
Materials and methods
32 children with CECTS and 25 age- and gender-matched healthy children were enrolled. All participants were imaged with 3.0-T MRI to acquire the structural and susceptibility-weighted data. The susceptibility-weighted data were processed using STISuite toolbox to obtain QSM. The magnetic susceptibility difference between the two groups was compared using voxel-wise and region of interest methods. Multivariable linear regression, controlling for age, were employed to investigate the associations between the brain magnetic susceptibility and age at onset.
Results
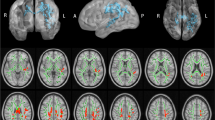
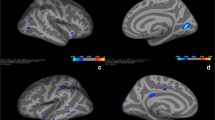
Lower magnetic susceptibility was mainly observed in sensory- and motor-related brain regions in children with CECTS, including bilateral middle frontal gyrus, supplementary motor area, midcingulate cortex, paracentral lobule and precentral gyrus, the magnetic susceptibility of right paracentral lobule, right precuneus and left supplementary motor area were found to have positive correlation with the age at onset.
Conclusions
This study suggests that the potential iron deficiency in certain brain regions is associated with CECTS, which might be helpful for further illumination of potential pathogenesis mechanism of CECTS.



Similar content being viewed by others
Abbreviations
- AAL:
-
Automated anatomical labeling
- ADHD:
-
Attention-deficit/hyperactivity disorder
- ALFF:
-
Amplitude of low-frequency fluctuation
- CECTS:
-
Childhood epilepsy with centrotemporal spikes
- EEG:
-
Electroencephalogram
- FEW:
-
Family-wise error
- HC:
-
Healthy controls
- MNI:
-
Montreal Neurological Institute
- QSM:
-
Quantitative susceptibility mapping
- SMA:
-
Supplementary motor area
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
References
Northcott E, Connolly AM, Berroya A, et al. Memory and phonological awareness in children with Benign Rolandic Epilepsy compared to a matched control group. Epilepsy Res. 2007;75:57–62.
Giordani B, Caveney AF, Laughrin D, et al. Cognition and behavior in children with benign epilepsy with centrotemporal spikes (bects). Epilepsy Res. 2006;70:89–94.
Nicolai J, Aldenkamp AP, Arends J, Weber JW, Vles JS. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2006;8:56–70.
Garcia-Ramos C, Jackson DC, Lin JJ, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56:1615–22.
Shakeri M, Datta AN, Malfait D, et al. Sub-cortical brain morphometry and its relationship with cognition in rolandic epilepsy. Epilepsy Res. 2017;138:39–45.
Tan G, Xiao F, Chen S, et al. Frequency-specific alterations in the amplitude and synchronization of resting-state spontaneous low-frequency oscillations in benign childhood epilepsy with centrotemporal spikes. Epilepsy Res. 2018;145:178–84.
Zeng H, Ramos CG, Nair VA, et al. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res. 2015;116:79–85.
Li R, Wang L, Chen H, et al. Abnormal dynamics of functional connectivity density in children with benign epilepsy with centrotemporal spikes. Brain Imaging Behav. 2019;13:985–94.
Xiao F, Chen Q, Yu X, et al. Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J Neurol Sci. 2014;336:171–9.
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–60.
Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–53.
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;25:863–73.
Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm (Vienna). 2011;118:301–14.
Jin L, Wang J, Zhao L, et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain. 2011;134:50–8.
Zhu WZ, Zhong WD, Wang W, et al. (2009) Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology. 2009;253:497–504.
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551–9.
Jang HN, Yoon HS, Lee EH. Prospective case control study of iron deficiency and the risk of febrile seizures in children in South Korea. BMC Pediatr. 2019;19:309–17.
Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with First-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31: 102736.
Faux NG, Rembach A, Wiley J, et al. An anemia of Alzheimer’s disease. Mol Psychiatry. 2014;19:1227–34.
Zhang Z, Liao W, Bernhardt B, et al. Brain iron redistribution in mesial temporal lobe epilepsy: a susceptibility-weighted magnetic resonance imaging study. BMC Neurosci. 2014;15:117–28.
Gorter JA, Mesquita AR, van Vliet EA, da Silva FH, Aronica E. Increased expression of ferritin, an iron-storage protein, in specific regions of the parahippocampal cortex of epileptic rats. Epilepsia. 2005;46:1371–9.
Wang Y, Liu T. Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2015;73:82–101.
Haacke EM, Liu S, Buch S, et al. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging. 2015;33:1–25.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51:676–85.
Li W, Avram AV, Wu B, Xiao X, Liu C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed. 2014;27:219–27.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34.
Bilgic B, Pfefferbaum A, Rohlfing T, et al. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage. 2012;59:2625–35.
Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2011;257:455–62.
Chen Y, Su S, Dai Y, et al. Quantitative susceptibility mapping reveals brain iron deficiency in children with attention-deficit/hyperactivity disorder: a whole-brain analysis. Eur Radiol. 2022;32:3726–33.
Tang S, Xu Y, Liu X, Chen Z, Zhou Y, Nie L, He L. Quantitative susceptibility mapping shows lower brain iron content in children with autism. Eur Radiol. 2021;31:2073–83.
Khattar N, Triebswetter C, Kiely M, et al. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. Neuroimage. 2021;239: 118267.
Zhang Q, He Y, Qu T, et al. Delayed brain development of Rolandic epilepsy profiled by deep learning-based neuroanatomic imaging. Eur Radiol. 2021;31:9628–37.
Cona G, Semenza C. Supplementary motor area as key structure for domain-general sequence processing: A unified account. Neurosci Biobehav Rev. 2017;72:28–42.
Zhu J, Xu C, Zhang X, et al. Altered topological properties of brain functional networks in drug-resistant epilepsy patients with vagus nerve stimulators. Seizure. 2021;92:149–54.
Qin Y, Jiang S, Zhang Q, et al. BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy. Neuroimage Clin. 2019;22: 101759.
Funding
This study has received funding by Guizhou Provincial Science and Technology Projects (grant number [2020]1Y347 and [2020] 1Y346).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Xu, G., Chen, X. & Zhang, Y. Quantitative susceptibility mapping shows lower brain iron content in children with childhood epilepsy with centrotemporal spikes. Jpn J Radiol 41, 1344–1350 (2023). https://doi.org/10.1007/s11604-023-01464-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-023-01464-5