Abstract
Objective
To explore the anti-inflammatory effects and mechanisms of action of thymol in Aspergillus fumigatus (A. fumigatus) keratitis.
Methods
The minimum inhibitory concentration of thymol against A. fumigatus was detected. To characterize the anti-inflammatory effects of thymol, mouse corneas and human corneal epithelial cells were pretreated with thymol or dimethyl sulfoxide (DMSO) before infection with A. fumigatus spores. Slit-lamp microscopy, immunohistochemistry, myeloperoxidase detection, quantitative real-time polymerase chain reaction, and Western blotting were used to assess infection. Neutrophil and macrophage recruitment, in addition to the secretion of LOX-1 and IL-1β, were quantified to evaluate the relative contribution of thymol to the inflammatory response.
Results
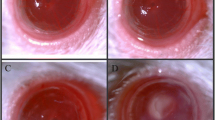
We confirmed that the growth of A. fumigatus was directly inhibited by thymol. In contrast with the DMSO group, there was a lower degree of inflammation in the mouse corneas of the thymol-pretreated group. This was characterized by significantly lower clinical scores, less inflammatory cell infiltration, and lower expression of LOX-1 and IL-1β. Similarly, in vitro experiments indicated that the production of LOX-1 and IL-1β was significantly inhibited after thymol treatment, in contrast with the DMSO-pretreated group.
Conclusion
Our findings demonstrate that thymol exerted a direct fungistatic activity on A. fumigatus. Furthermore, thymol played a protective role in fungal keratitis by inhibiting LOX-1/IL-1β signaling pathway and reducing the recruitment of neutrophils and macrophages.
Similar content being viewed by others
References
Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol, 2007,14(2):61–69
Srinivasan M, Christine A, Celine G, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol, 1997,81:965–971
Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol, 2015,37(2):97–106
Figueiredo RT, Carneiro LA, Bozza MT. Fungal surface and innate immune recognition of filamentous fungi. Front Microbiol, 2011,2:248
Menda SA, Das M, Panigrahi A, et al. Association of Postfungal Keratitis Corneal Scar Features With Visual Acuity. JAMA Ophthalmology, 2020,138(2):113–118
Jeng BH. Challenges in the Management of Fungal Keratitis. JAMA Ophthalmology, 2017,135(6):525–526
Mancini E, Senatore F, Del Monte D, et al. Studies on Chemical Composition, Antimicrobial and Antioxidant Activities of Five Thymus vulgaris L. Essential Oils. Molecules, 2015,20(7):12 016–12 028
Marchese A, Orhan IE, Daglia M, et al. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chemistry, 2016,210:402–414
Mendes SS, Bomfim RR, Jesus HC, et al. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J Ethnopharmacol, 2010,129(3):391–397
Wang K, Jiang S, Pu T, et al. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat Prod Res, 2019,33(10):1423–1430
Sharifzadeh A, Khosravi AR, Shokri H, et al. Potential effect of 2-isopropyl-5-methylphenol (thymol) alone and in combination with fluconazole against clinical isolates of Candida albicans, C. glabrata and C. krusei. J Mycol Med, 2018,28(2):294–299
Mathela CS, Singh KK, Gupta VK. Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Pol Pharm, 2010,67(4):375–380
Haeseler G, Maue D, Grosskreutz J, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol, 2002,19:571–579
Aeschbach R, Löliger R, Scott BC, et al. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol, 1994,32(1):31–36
Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci, 2003,44(1):210–216
Manikandan P, Abdel-Hadi A, Randhir Babu Singh Y, et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. BioMed Res Int, 2019,2019:6395840
Yang H, Wang Q, Han L, et al. Nerolidol inhibits the LOX-1/IL-1beta signaling to protect against the Aspergillus fumigatus keratitis inflammation damage to the cornea. Int Immunopharmacol, 2020,80:106118
Rose-Nussbaumer J, Prajna NV, Krishnan T, et al. Mycotic Ulcer Treatment Trial, Risk factors for low vision related functioning in the Mycotic Ulcer Treatment Trial: a randomised trial comparing natamycin with voriconazole. Br J Ophthalmol, 2016,100(7):929–932
Bae YS, Rhee MS. Short-Term Antifungal Treatments of Caprylic Acid with Carvacrol or Thymol Induce Synergistic 6-Log Reduction of Pathogenic Candida albicans by Cell Membrane Disruption and Efflux Pump Inhibition. Cell Physiol Biochem, 2019,53(2):285–300
Braga PC, Culici M, Alfieri M, et al. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents, 2008,31(5):472–477
Zhang M, Ge J, Yu X. Transcriptome Analysis Reveals the Mechanism of Fungicidal of Thymol Against Fusarium oxysporum f. sp. niveum. Curr Microbiol, 2018,75(4):410–419
Kasper L, Konig A, Koenig PA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun, 2018,9(1):4260
Golbahari S, Abtahi Froushani SM. Synergistic benefits of Nicotine and Thymol in alleviating experimental rheumatoid arthritis. Life Sci, 2019,239:117037
Eftekhari M, Hoseinsalari A, Mansourian M, et al. Trachyspermum ammi (L.) Sprague, superb essential oil and its major components on peptic ulcers: in vivo combined in silico studies. DARU J Pharm Sci, 2019, 27(1):317–327
Yao L, Hou G, Wang L, et al. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb Pathog, 2018,116:8–12
Hind LE, Huttenlocher A. Neutrophil Reverse Migration and a Chemokinetic Resolution. Dev Cell, 2018,47(4): 404–405
Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol, 2005,6(12): 1191–1197
Boff D, Crijns H, Teixeira MM, et al. Neutrophils: beneficial and harmful cells in septic arthritis. Int J Mol Sci, 2018,19(2):468
Niu L, Liu X, Ma Z, et al. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb Pathog, 2020,138:103802
Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J, 2007,21(2):325–332
Hu JZ, Hu YF, Chen SK, et al. Role of activated macrophages in experimental Fusarium solani keratitis. Exp Eye Res, 2014,129:57–65
Zhang Q, Liu J, Ma L, et al. Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory response in peri-implantitis. J Periodontal Res, 2020,55(2):199–208
Zhang Q, Liu J, Ma L, et al. LOX-1 is involved in TLR2 induced RANKL regulation in peri-implantitis. Int Immunopharmacol, 2019,77:105956
Li C, Zhao GQ, Che CY, et al. The Role of LOX-1 in Innate Immunity to Aspergillus fumigatus in Corneal Epithelial Cells. Invest Ophthalmol Vis Sci, 2015,56(6): 3593–3603
Che CY, Li C, Lin J, et al. Wnt5a contributes to dectin-1 and LOX-1 induced host inflammatory response signature in Aspergillus fumigatus keratitis. Cell Signal, 2018,52:103–111
Palomo J, Dietrich D, Martin P, et al. The interleukin (IL)-1 cytokine family—Balance between agonists and antagonists in inflammatory diseases. Cytokine, 2015, 76(1):25–37
Zhao GQ, Hu M, Li C, et al. Osteopontin contributes to effective neutrophil recruitment, IL-1beta production and apoptosis in Aspergillus fumigatus keratitis. Immunol Cell Biol, 2018,96(4):401–412
Cheng M, Lin J, Li C, et al. Wedelolactone suppresses IL-1beta maturation and neutrophil infiltration in Aspergillus fumigatus keratitis. Int Immunopharmacol, 2019,73:17–22
Pearlman E, Sun Y, Roy S, et al. Host defense at the ocular surface. Int Rev Immunol, 2013,32(1):4–18
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The authors have no conflicts of interest.
This project was supported by grants from the National Natural Science Foundation of China (No. 82171019), Natural Science Foundation of Shandong Province (No. ZR2021MH368), Traditional Chinese Medicine Research Project of Qingdao (No. 2020-zyy055) and Shandong Qingdao Outstanding Health Professional Development Fund.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wang, Lm., Yang, H., Yan, Hj. et al. Thymol Protects against Aspergillus Fumigatus Keratitis by Inhibiting the LOX-1/IL-1β Signaling Pathway. CURR MED SCI 42, 620–628 (2022). https://doi.org/10.1007/s11596-022-2512-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2512-9