Summary
Tumorigenicity-inhibiting compounds have been identified in our daily diet. For example, isothiocyanates (ITCs) found in cruciferous vegetables were reported to have potent cancer-prevention activities. The best characterized ITC is sulforaphane (SF). SF can simultaneously modulate multiple cellular targets involved in carcinogenesis, including (1) modulating carcinogen-metabolizing enzymes and blocking the action of mutagens; (2) inhibition of cell proliferation and induction of apoptosis; and (3) inhibition of neo-angiogenesis and metastasis. SF targets cancer stem cells through modulation of nuclear factor kappa B (NF-κB), Sonic hedgehog (SHH), epithelial-mesenchymal transition, and Wnt/β-catenin pathways. Conventional chemotherapy/SF combination was tested in several studies and resulted in favorable outcomes. With its favorable toxicological profile, SF is a promising agent in cancer prevention and/or therapy. In this article, we discuss the human metabolism of SF and its effects on cancer prevention, treatment, and targeting cancer stem cells, as well as providing a brief review of recent human clinical trials on SF.
Similar content being viewed by others
References
von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs, 2005,9(Suppl 2):S35–38
Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer, 2003,3(10):768–780
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell, 2011,144(5):646–674
Mithen R, Faulkner K, Magrath R, et al. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor Appl Genet, 2003,106(4):727–734
Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res, 2007, 55(3):224–236
Verhoeven DT, Goldbohm RA, van Poppel G, et al. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev, 1996, 5(9):733–748
Voorrips LE, Goldbohm RA, Verhoeven DT, et al. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes Control, 2000,11(2):101–115
Voorrips LE, Goldbohm RA, van Poppel G, et al. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol, 2000,152(11):1081–1092
Feskanich D, Ziegler RG, Michaud DS, et al. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst, 2000,92(22):1812–1823
Giovannucci E, Rimm EB, Liu Y, et al. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev, 2003,12(12):1403–1409
Joseph MA, Moysich KB, Freudenheim JL, et al. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer, 2004,50(2):206–213
Neuhouser ML, Patterson RE, Thornquist MD, et al. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev, 2003,12(4):350–358
Jeffery EH, Keck AS. Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res, 2008,52(Suppl 1):S7–17
Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr, 2000,72(6):1424–1435
Bianchini F, Vainio H. Isothiocyanates in cancer prevention. Drug Metab Rev, 2004,36(3–4):655–667
Tortorella SM, Royce SG, Licciardi PV, et al. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid Redox Signal, 2015,22(16):1382–1424
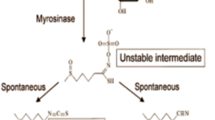
Bell L, Wagstaff C. Glucosinolates, Myrosinase Hydrolysis Products, and Flavonols Found in Rocket (Eruca sativa and Diplotaxis tenuifolia). J Agric Food Chem, 2014,62(20):4481–4492
Conaway CC, Krzeminski J, Amin S, et al. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem Res Toxicol, 2001,14(9):1170–1176
Shapiro TA, Fahey JW, Wade KL, et al. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev, 1998,7(12):1091–1100
Matusheski NV, Jeffery EH. Comparison of the Bioactivity of Two Glucoraphanin Hydrolysis Products Found in Broccoli, Sulforaphane and Sulforaphane Nitrile. J Agric Food Chem, 2001,49(12):5743–5749
Liang H, Yuan Q. Natural sulforaphane as a functional chemopreventive agent: including a review of isolation, purification and analysis methods. Crit Rev Biotechnol, 2012,32(3):218–234
Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A, 1992,89(6):2394–2398
West LG, Meyer KA, Balch BA, et al. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J Agric Food Chem, 2004,52(4):916–926
Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer, 2011,63(2):196–201
Clarke JD, Hsu A, Riedl K, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res, 2011,64(5):456–463
Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci, 2007,64(9):1105–1127
Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A, 1997,94(19):10367–10372
Prakash B, Amuthavalli A, Edison D, et al. Novel indole derivatives as potential anticancer agents: Design, synthesis and biological screening. Med Chem Res, 2018,27(1):321–331
Petri N, Tannergren C, Holst B, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos, 2003,31(6):805–813
Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer, 2000,38(2):168–178
Rungapamestry V, Duncan AJ, Fuller Z, et al. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc Nutr Soc, 2007,66(1):69–81
Vermeulen M, Klopping-Ketelaars IW, van den Berg R, et al. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem, 2008,56(22):10505–10509
Jones RB, Frisina CL, Winkler S, et al. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem, 2010,123(2):237–242
Saha S, Hollands W, Teucher B, et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res, 2012,56(12):1906–1916
Wang GC, Farnham M, Jeffery EH. Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica). J Agric Food Chem, 2012,60(27):6743–6748
Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochermcal. Acta Pharmacol Sin, 2007,28:1343
Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis, 2000,21(6):1175–1182
Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis, 2009,31(1):90–99
Zhang Y, Kolm RH, Mannervik B, et al. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun, 1995,206(2):748–755
Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J, 2002,364(Pt 1):301–307
Callaway EC, Zhang Y, Chew W, et al. Cellular accumulation of dietary anticarcinogenic isothiocyanates is followed by transporter-mediated export as dithiocarbamates. Cancer Lett, 2004,204(1):23–31
Brüsewitz G, Cameron BD, Chasseaud LF, et al. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem J, 1977,162(1):99–107
Herr I, Buchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev, 2010,36(5):377–383
Cramer JM, Teran-Garcia M, Jeffery EH. Enhancing sulforaphane absorption and excretion in healthy men through the combined consumption of fresh broccoli sprouts and a glucoraphanin-rich powder. Br J Nutr, 2012,107(9):1333–1338
Al Janobi AA, Mithen RF, Gasper AV, et al. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2006,844(2):223–234
Ye L, Dinkova-Kostova AT, Wade KL, et al. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta, 2002,316(1–2):43–53
Hu R, Hebbar V, Kim B-R, et al. In Vivo Pharmacokinetics and Regulation of Gene Expression Profiles by Isothiocyanate Sulforaphane in the Rat. J Pharmacol Exp Ther, 2004,310(1):263–271
Khor TO, Hu R, Shen G, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Cancer Res, 2006,66(8 Supplement):1316–1316
Cornblatt BS, Ye L, Dinkova-Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis, 2007,28(7):1485–1490
Lock EA, Reed CJ. Xenobiotic metabolizing enzymes of the kidney. Toxicol Pathol, 1998,26(1):18–25
Tang L, Li G, Song L, et al. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs, 2006,17(3):297–305
Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a Promising Molecule for Fighting Cancer. In Proceedings of Advances in Nutrition and Cancer, Berlin, Heidelberg, 2014:207–223
Iyanagi T. Molecular Mechanism of Phase I and Phase II Drug-Metabolizing Enzymes: Implications for Detoxification. In International Review of Cytology, Academic Press, 2007,260:35–112
Deng J, Zhao L, Zhang NY, et al. Anatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res, 2018,778:79–89
Barcelo S, Gardiner JM, Gescher A, et al. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis, 1996,17(2): 277–282
Barcelo S, Mace K, Pfeifer AM, et al. Production of DNA strand breaks by N-nitrosodimethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforaphane. Mutat Res, 1998,402(1–2):111–120
Bacon JR, Williamson G, Garner RC, et al. Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis, 2003,24(12):1903–1911
Rendic S, Guengerich FP. Contributions of Human Enzymes in Carcinogen Metabolism. Chem Res Toxicol, 2012,25(7):1316–1383
Maheo K, Morel F, Langouet S, et al. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res, 1997,57(17):3649–3652
Gross-Steinmeyer K, Stapleton PL, Tracy JH, et al. Sulforaphane- and phenethyl isothiocyanate-induced inhibition of aflatoxin B1-mediated genotoxicity in human hepatocytes: role of GSTM1 genotype and CYP3A4 gene expression. Toxicol Sci, 2010,116(2):422–432
Zhou C, Poulton EJ, Grun F, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol, 2007,71(1):220–229
Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect, 1997,105(Suppl 4):965–970
Vargas-Mendoza N, Morales-Gonzalez A, Madrigal-Santillan EO, et al. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants (Basel), 2019,8(6):196
Chen Y, Shertzer HG, Schneider SN, et al. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem, 2005,280(40):33766–33774
Hu R, Xu C, Shen G, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett, 2006,243(2): 170–192
Thimmulappa RK, Mai KH, Srisuma S, et al. Identification of Nrf2-regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray. Cancer Res, 2002,62(18): 5196–5203
Wu L, Juurlink BH. The impaired glutathione system and its up-regulation by sulforaphane in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens, 2001,19(10):1819–1825
Jiang ZQ, Chen C, Yang B, et al. Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers. Life Sci, 2003,72(20):2243–2253
Basten GP, Bao Y, Williamson G. Sulforaphane and its glutathione conjugate but not sulforaphane nitrile induce UDP-glucuronosyl transferase (UGT1A1) and glutathione transferase (GSTA1) in cultured cells. Carcinogenesis, 2002,23(8):1399–1404
Zhang Y, Gordon GB. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther, 2004,3(7):885–893
Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol, 2005,18(12):1779–1791
Kensler TW, Egner PA, Agyeman AS, et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem, 2013,329:163–177
Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol, 2005,18(12):1917–1926
Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A, 2002,99(18):11908–11913
Tao S, Rojo de la Vega M, Chapman E, et al. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol Carcinog, 2018,57(2):182–192
Shorey LE, Madeen EP, Atwell LL, et al. Differential modulation of dibenzo[def, p]chrysene transplacental carcinogenesis: maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol Appl Pharmacol, 2013,270(1):60–69
Shishu, Kaur IP. Inhibition of mutagenicity of food-derived heterocyclic amines by sulforaphane—a constituent of broccoli. Indian J Exp Biol, 2003,41(3): 216–219
Singletary K, MacDonald C. Inhibition of benzo[a] pyrene- and 1,6-dinitropyrene-DNA adduct formation in human mammary epithelial cells bydibenzoylmethane and sulforaphane. Cancer Lett, 2000,155(1):47–54
Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res, 2001,61(16):6120–6130
Fimognari C, Berti F, Cantelli-Forti G, et al. Effect of sulforaphane on micronucleus induction in cultured human lymphocytes by four different mutagens. Environ Mol Mutagen, 2005,46(4):260–267
Gills JJ, Jeffery EH, Matusheski NV, et al. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett, 2006,236(1):72–79
Kuroiwa Y, Nishikawa A, Kitamura Y, et al. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett, 2006,241(2):275–280
Chiao JW, Chung FL, Kancherla R, et al. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol, 2002,20(3): 631–636
Shan Y, Sun C, Zhao X, et al. Effect of sulforaphane on cell growth, G(0)/G(1) phase cell progression and apoptosis in human bladder cancer T24 cells. Int J Oncol, 2006,29(4):883–888
Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr, 2004,134(8):2004–2010
Jackson SJT, Singletary KW. Sulforaphane Inhibits Human MCF-7 Mammary Cancer Cell Mitotic Progression and Tubulin Polymerization. Journal Nutr, 2004,134(9):2229–2236
Parnaud G, Li P, Cassar G, et al. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer, 2004,48(2):198–206
Pham N-A, Jacobberger JW, Schimmer AD, et al. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther, 2004,3(10):1239–1248
Singh SV, Herman-Antosiewicz A, Singh AV, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem, 2004,279(24):25813–25822
Wang L, Liu D, Ahmed T, et al. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol, 2004,24(1):187–192
Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res, 2000,60(5):1426–1433
Wang M, Chen S, Wang S, et al. Effects of phytochemicals sulforaphane on uridine diphosphate-glucuronosyltransferase expression as well as cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Chin J Physiol, 2012,55(2):134–144
Kim MR, Zhou L, Park BH, et al. Induction of G(2)/M arrest and apoptosis by sulforaphane in human osteosarcoma U2-OS cells. Mol Med Rep, 2011,4(5): 929–934
Kim JH, Han Kwon K, Jung JY, et al. Sulforaphane Increases Cyclin-Dependent Kinase Inhibitor, p21 Protein in Human Oral Carcinoma Cells and Nude Mouse Animal Model to Induce G(2)/M Cell Cycle Arrest. J Clin Biochem Nutr, 2010,46(1):60–67
Suppipat K, Park CS, Shen Y, et al. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS One, 2012,7(12):e51251–e51251
Jakubikova J, Cervi D, Ooi M, et al. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and phenethyl isothiocyanate, in multiple myeloma. Haematologica, 2011,96(8):1170–1179
Jackson SJT, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis, 2004,25(2):219–227
Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell, 2003,3(1):17–22
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol, 2007,35(4):495–516
Gamet-Payrastre L, Lumeau S, Gasc N, et al. Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro. Anticancer Drugs, 1998,9(2):141–148
Jakubíková J, Sedlák J, Mithen R, et al. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol, 2005,69(11):1543–1552
Choi S, Lew KL, Xiao H, et al. D, L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis, 2007,28(1):151–162
Singh AV, Xiao D, Lew KL, et al. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis, 2004,25(1):83–90
Gingras D, Gendron M, Boivin D, et al. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett, 2004,203(1):35–43
Chaudhuri D, Orsulic S, Ashok BT. Antiproliferative activity of sulforaphane in Akt-overexpressing ovarian cancer cells. Mol Cancer Ther, 2007,6(1):334–345
Fimognari C, Nusse M, Berti F, et al. Sulforaphane modulates cell cycle and apoptosis in transformed and non-transformed human T lymphocytes. Ann N Y Acad Sci, 2003,1010:393–398
Fimognari C, Nusse M, Cesari R, et al. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis, 2002,23(4):581–586
Misiewicz I, Skupinska K, Kasprzycka-Guttman T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol Rep, 2003,10(6):2045–2050
Pappa G, Lichtenberg M, Iori R, et al. Comparison of growth inhibition profiles and mechanisms of apoptosis induction in human colon cancer cell lines by isothiocyanates and indoles from Brassicaceae. Mutat Res, 2006,599(1–2):76–87
Park HS, Han MH, Kim GY, et al. Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol, 2014,64:157–165
Park SY, Kim GY, Bae SJ, et al. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol Rep, 2007,18(1): 181–187
Devi JR, Thangam EB. Mechanisms of anticancer activity of sulforaphane from Brassica oleracea in HEp-2 human epithelial carcinoma cell line. Asian Pac J Cancer Prev, 2012,13(5):2095–2100
Cho NP, Han HS, Leem DH, et al. Sulforaphane enhances caspase-dependent apoptosis through inhibition of cyclooxygenase-2 expression in human oral squamous carcinoma cells and nude mouse xenograft model. Oral Oncol, 2009,45(8):654–660
Cho SD, Li G, Hu H, et al. Involvement of c-Jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by sulforaphane in DU145 prostate cancer cells. Nutr Cancer, 2005,52(2):213–224
Singh SV, Srivastava SK, Choi S, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem, 2005, 280(20):19911–19924
Moon DO, Kim MO, Kang SH, et al. Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett, 2009,274(1):132–142
Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res, 2006,66(11):5828–5835
Jeong WS, Kim IW, Hu R, et al. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res, 2004,21(4):661–670
Xu C, Shen G, Chen C, et al. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene, 2005,24(28):4486–4495
Ramirez MC, Singletary K. Regulation of estrogen receptor alpha expression in human breast cancer cells by sulforaphane. J Nutr Biochem, 2009,20(3):195–201
Kim H, Kim EH, Eom YW, et al. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res, 2006,66(3):1740–1750
Matsui TA, Sowa Y, Yoshida T, et al. Sulforaphane enhances TRAIL-induced apoptosis through the induction of DR5 expression in human osteosarcoma cells. Carcinogenesis, 2006,27(9):1768–1777
Myzak MC, Karplus PA, Chung FL, et al. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res, 2004, 64(16):5767–5774
Myzak MC, Hardin K, Wang R, et al. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis, 2006,27(4):811–819
Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst, 2000,92(15):1210–1216
Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A, 2005,102(3):673–678
Tortorella SM, Royce SG, Licciardi PV, et al. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid Redox Signal, 2015,22(16):1382–1424
Cuervo AM. Autophagy: Many paths to the same end. Mol Cell Biochem, 2004,263(1):55–72
Jia L, Dourmashkin RR, Allen PD, et al. Inhibition of autophagy abrogates tumour necrosis factor α induced apoptosis in human T-lymphoblastic leukaemic cells. Br J Haematol, 1997,98(3):673–685
Kanematsu S, Uehara N, Miki H, et al. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res, 2010,30(9):3381–3390
Beaver LM, Kuintzle R, Buchanan A, et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem, 2017,42:72–83
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature, 2000,407:249
Goldmann E. The growth of malignant disease in main and the lower animals: with special reference to the vascular system. Lancet, 1907,170(4392):1236–1240
Algire GH, Chalkley HW, Legallais FY, et al. Vasculae Reactions of Normal and Malignant Tissues in Vivo. I. Vascular Reactions of Mice to Wounds and to Normal and Neoplastic Transplants. J Natl Cancer Inst, 1945,6(1):73–85
Ide AG. Vascularization of the Brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. AJR Am J Roentgenol, 1939,42:891–899
Greenblatt M, Philippe SK. Tumor Angiogenesis: Transfilter Diffusion Studies in the Hamster by the Transparent Chamber Technique2. J Natl Cancer Inst, 1968,41(1):111–124
Ehrmann RL, Knoth M. Choriocarcinoma: Transfilter Stimulation of Vasoproliferation in the Hamster Cheek Pouch—Studied by Light and Electron Microscopy2. J Natl Cancer Inst, 1968,41(6):1329–1341
Gupta GK, Milner L, Linshaw MA, et al. Urinary basic fibroblast growth factor: A noninvasive marker of progressive cystic renal disease in a child. Am J Med Genet, 2000,93(2):132–135
Nishida N, Yano H, Nishida T, et al. Angiogenesis in cancer. Vasc Health Risk Manag, 2006,2(3):213–219
Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res, 2014,159:207–223
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology, 2005,69(Suppl 3):4–10
Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther, 2006,5(3):575–585
Bao Y, Wang W, Zhou Z, et al. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS One, 2014,9(12):e114764–e114764
Asakage M, Tsuno NH, Kitayama J, et al. Sulforaphane induces inhibition of human umbilical vein endothelial cells proliferation by apoptosis. Angiogenesis, 2006, 9(2):83–91
Jackson SJ, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascul Pharmacol, 2007,46(2):77–84
Netland PA, Zetter BR. Tumor-Cell Interactions with Blood Vessels during Cancer Metastasis. In Fundamental Aspects of Cancer, Goldfarb RH, Ed. Springer Netherlands: Dordrecht, 1989:84–97
Liotta LA. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol, 1984,117(3):339–348
Fidler IJ. Tumor Heterogeneity and the Biology of Cancer Invasion and Metastasis. Cancer Res, 1978, 38(9):2651–2660
Liotta LA. Tumor invasion and metastases—role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res, 1986,46(1):1–7
Liotta LA, Tryggvason K, Garbisa S, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature, 1980,284:67
Nicolson GL. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta, 1982,695(2):113–176
Thejass P, Kuttan G. Antimetastatic activity of Sulforaphane. Life Sci, 2006,78(26):3043–3050
Chambers AF, Matrisian LM. Changing Views of the Role of Matrix Metalloproteinases in Metastasis. J Natl Cancer Inst, 1997,89(17):1260–1270
Stetler-Stevenson WG, Hewitt R, Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol, 1996, 7(3):147–154
Haizhen Z, Bowen JP. Antiangiogenesis Drug Design: Multiple Pathways Targeting Tumor Vasculature. Curr Med Chem, 2006,13(8):849–862
Jee HG, Lee KE, Kim JB, et al. Sulforaphane Inhibits Oral Carcinoma Cell Migration and Invasion In Vitro. Phytother Res, 2011,25(11):1623–1628
Kanematsu S, Yoshizawa K, Uehara N, et al. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol Rep, 2011,26(3):603–608
Wang DX, Zou YJ, Zhuang XB, et al. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3beta/beta-catenin signaling pathways. Acta Pharmacol Sin, 2017,38(2):241–251
Bouhassira EE. The SAGE Encyclopedia of Stem Cell Research. Second Edition ed. Thousand Oaks, California, 2015, https://doi.org/10.4135/9781483347660
Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature, 2001,414:105
Li Y, Zhang T. Targeting cancer stem cells with sulforaphane, a dietary component from broccoli and broccoli sprouts. Future Oncol, 2013,9(8):1097–1103
Kallifatidis G, Rausch V, Baumann B, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut, 2009,58(7):949–963
Tang SN, Fu J, Nall D, et al. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer, 2012,131(1):30–40
Munoz P, Iliou MS, Esteller M. Epigenetic alterations involved in cancer stem cell reprogramming. Mol Oncol, 2012,6(6):620–636
Canettieri G, Di Marcotullio L, Greco A, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol, 2010,12(2):132–142
Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res, 2006,66(14):7041–7049
Rodova M, Fu J, Watkins DN, et al. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One, 2012,7(9):e46083
Li SH, Fu J, Watkins DN, et al. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem, 2013,373(1–2):217–227
Ali Khan M, Kedhari Sundaram M, Hamza A, et al. Sulforaphane Reverses the Expression of Various Tumor Suppressor Genes by Targeting DNMT3B and HDAC1 in Human Cervical Cancer Cells. Evid Based Complement Alternat Med, 2015,2015:412149
Iwatsuki M, Mimori K, Yokobori T, et al. Epithelialmesenchymal transition in cancer development and its clinical significance. Cancer Sci, 2010,101(2):293–299
Liu X, Fan D. The epithelial-mesenchymal transition and cancer stem cells: functional and mechanistic links. Curr Pharm Des, 2015,21(10):1279–1291
Srivastava RK, Tang SN, Zhu W, et al. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed), 2011,3:515–528
Shan Y, Zhang L, Bao Y, et al. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J Nutr Biochem, 2013, 24(6):1062–1069
Fresno Vara JA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev, 2004,30(2):193–204
Shen G, Khor TO, Hu R, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res, 2007,67(20):9937–9944
Valkenburg KC, Graveel CR, Zylstra-Diegel CR, et al. Wnt/β-catenin Signaling in Normal and Cancer Stem Cells. Cancers (Basel), 2011,3(2):2050–2079
Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell, 2007,1(5):555–567
Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res, 2010,16(9):2580–2590
Kato M, Slack FJ. microRNAs: small molecules with big roles—C. elegans to human cancer. Biol Cell, 2008,100(2):71–81
Yu SL, Chen HY, Chang GC, et al. MicroRNA Signature Predicts Survival and Relapse in Lung Cancer. Cancer Cell, 2008,13(1):48–57
Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA, 2009,106(29):12085–12090
Iorio MV, Casalini P, Tagliabue E, et al. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer, 2008,44(18):2753–2759
Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA, 2002,99(24):15524–15529
Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene, 2006,25:6202
Ventura A, Jacks T. MicroRNAs and Cancer: Short RNAs Go a Long Way. Cell, 2009,136(4):586–591
Li J, Huang H, Sun L, et al. MiR-21 Indicates Poor Prognosis in Tongue Squamous Cell Carcinomas as an Apoptosis Inhibitor. Clin Cancer Res, 2009,15(12):3998–4008
Liu CJ, Tsai MM, Hung PS, et al. miR-31 Ablates Expression of the HIF Regulatory Factor FIH to Activate the HIF Pathway in Head and Neck Carcinoma. Cancer Res, 2010,70(4):1635–1644
Yang MH, Lin BR, Chang CH, et al. Connective tissue growth factor modulates oral squamous cell carcinoma invasion by activating a miR-504/FOXP1 signalling. Oncogene, 2011,31:2401
Lu YC, Chen YJ, Wang HM, et al. Oncogenic Function and Early Detection Potential of miRNA-10b in Oral Cancer as Identified by microRNA Profiling. Cancer Prev Res, 2012,5(4):665–674
Yu CC, Chen YW, Chiou GY, et al. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol, 2011,47(3):202–210
Liu CM, Peng CY, Liao YW, et al. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J Formos Med Assoc, 2017,116(1):41–48
Li Q, Yao Y, Eades G, et al. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene, 2013,33:2589
Li X, Zhao Z, Li M, et al. Sulforaphane promotes apoptosis, and inhibits proliferation and self-renewal of nasopharyngeal cancer cells by targeting STAT signal through miRNA-124-3p. Biomed Pharmacother, 2018,103:473–481
Kallifatidis G, Labsch S, Rausch V, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther, 2011,19(1):188–195
Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest, 2011,121(1):396–409
Lin LC, Yeh CT, Kuo CC, et al. Sulforaphane Potentiates the Efficacy of Imatinib against Chronic Leukemia Cancer Stem Cells through Enhanced Abrogation of Wnt/β-Catenin Function. J Agric Food Chem, 2012,60(28):7031–7039
Chen H, Landen CN, Li Y, et al. Enhancement of Cisplatin-Mediated Apoptosis in Ovarian Cancer Cells through Potentiating G2/M Arrest and p21 Upregulation by Combinatorial Epigallocatechin Gallate and Sulforaphane. J Oncol, 2013,2013:9
Wang X, Li Y, Dai Y, et al. Sulforaphane improves chemotherapy efficacy by targeting cancer stem celllike properties via the miR-124/IL-6R/STAT3 axis. Sci Rep, 2016,6:36796
Elkashty OA, Ashry R, Elghanam GA, et al. Broccoli extract improves chemotherapeutic drug efficacy against head-neck squamous cell carcinomas. Med Oncol, 2018,35(9):124
Fimognari C, Nusse M, Lenzi M, et al. Sulforaphane increases the efficacy of doxorubicin in mouse fibroblasts characterized by p53 mutations. Mutat Res, 2006,601(1–2):92–101
Fimognari C, Lenzi M, Sciuscio D, et al. Combination of doxorubicin and sulforaphane for reversing doxorubicinresistant phenotype in mouse fibroblasts with p53Ser220 mutation. Ann N Y Acad Sci, 2007,1095:62–69
Kaminski BM, Steinhilber D, Stein JM, et al. Phytochemicals resveratrol and sulforaphane as potential agents for enhancing the anti-tumor activities of conventional cancer therapies. Curr Pharm Biotechnol, 2012,13(1):137–146
Dosz EB, Jeffery EH. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J Funct Foods, 2013,5(2):987–990
Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev, 2013,71(11):709–726
Cipolla BG, Mandron E, Lefort JM, et al. Effect of Sulforaphane in Men with Biochemical Recurrence after Radical Prostatectomy. Cancer Prev Res, 2015,8(8):712–719
Kensler TW, Chen JG, Egner PA, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev, 2005,14(11 Pt 1):2605–2613
Egner PA, Chen JG, Wang JB, et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila), 2011,4(3):384–395
Kensler TW, Ng D, Carmella SG, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis, 2012,33(1):101–107
Fahey JW, Wehage SL, Holtzclaw WD, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila), 2012,5(4):603–611
Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol, 2009,130(3):244–251
Alumkal JJ, Slottke R, Schwartzman J, et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs, 2015,33(2):480–489
Broccoli Sprout Extract in Preventing Recurrence in Patients With Tobacco-Related Head and Neck Squamous Cell Cancer. Availabe online: https://ClinicalTrials.gov/show/NCT03182959 (accessed on May 2002)
Myzak MC, Tong P, Dashwood WM, et al. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood), 2007,232(2):227–234
Traka M, Gasper AV, Melchini A, et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS One, 2008,3(7):e2568
Hanlon N, Coldham N, Gielbert A, et al. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett, 2009,284(1):15–20
Bahadoran Z, Mirmiran P, Hosseinpanah F, et al. Broccoli sprouts reduce oxidative stress in type 2 diabetes: a randomized double-blind clinical trial. Eur J Clin Nutr, 2011,65(8):972–977
Zhang Z, Garzotto M, Davis EW 2nd, et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr Cancer, 2020,72(1):74–87
Study to Evaluate the Effect of Sulforaphane in Broccoli Sprout Extract on Breast Tissue. Availabe online: https://clinicaltrials.gov/ct2/show/study/NCT00982319 (accessed on May 2020).
Tahata S, Singh SV, Lin Y, et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev Res (Phila), 2018,11(7):429–438
Traka MH, Melchini A, Coode-Bate J, et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate Cancer Prevention (ESCAPE) randomized controlled trial. Am J Clin Nutr, 2019,109(4):1133–1144
Atwell LL, Zhang Z, Mori M, et al. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev Res (Phila), 2015,8(12):1184–1191
Protective Effects of the Nutritional Supplement Sulforaphane on Doxorubicin-Associated Cardiac Dysfunction. Availabe online: https://clinicaltrials.gov/ct2/show/NCT03934905?term=sulforaphane&rank=1 (accessed on May 2020).
Suh N, Luyengi L, Fong HH, et al. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model. Anticancer Res, 1995,15(2):233–239
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
Authors declare no conflict of interest in the study.
Rights and permissions
About this article
Cite this article
Elkashty, O.A., Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. CURR MED SCI 41, 250–269 (2021). https://doi.org/10.1007/s11596-021-2341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2341-2