Summary
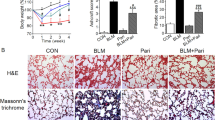
The dynamic variation of renin-angiotensin system (RAS) in silicosis remains unclear. Seventy Wistar rats were divided into 7 groups including control group, silicosis groups (inhaling SiO2 for 2, 4, 8, 16 and 24 weeks, respectively) and Captopril (Cap) group. Rat lung primary fibroblasts were divided into control group, SiO2-stimulated group (0, 0.5, 1, 3, 6, 12, 24 and 48 h) and Cap group. The silicotic nodules were formed and collagens were deposited gradually in silicosis group observed by haematoxylin and eosin (HE) staining and Van Gieson (VG) staining. Cap relieved the lung fibrosis and collagen deposition. Immunohistochemistry indicated the positive expression of α-smooth muscle actin (α-SMA) was increased gradually in silicotic rat lung tissue. Western blotting revealed the expression of collagen type I (Col I) and α-SMA was up-regulated in silicotic rat lung tissue and fibroblasts stimulated by SiO2. Cap decreased the expression of Col I and α-SMA in silicotic rat lung tissue and fibroblasts stimulated by SiO2. Western blotting also demonstrated the expression of angiotensin-converting enzyme (ACE) and angiotensin II type 1 receptor (AT1) was increased, and the expression of ACE2 and Mas was decreased gradually in silicotic rat lung tissue and fibroblasts stimulated by SiO2. ELISA showed the serum levels of ACE and angiotensin II (Ang II) were also increased and ACE2 and Ang (1-7) were decreased in the silicosis group. Treatment with Cap decreased the expression levels of ACE, Ang II and AT1, and increased the expression levels of ACE2, Ang (1-7) and Mas. These findings suggested that an imbalance between ACE-Ang II-AT1 axis and ACE2-Ang (1-7)-Mas axis may participate in the development of silicosis.
Similar content being viewed by others
References
Stanbury, M, Rosenman, KD. Occupational health disparities: a state public health based approach. Am J Ind Med, 2014,57(5):596–604
Jiang, CQ, Xiao, LW, Lam, TH, et al. Accelerated silicosis in workers exposed to agate dust in Guangzhou, China. Am J Ind Med, 2001,40(1):87–91
Rimal, B, Greenberg, AK, Rom, WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med, 2005,11(2):169–173
Wang, J, Chen, L, Chen, B, et al. Chronic Activation of the Renin-Angiotensin System Induces Lung Fibrosis. Sci Rep, 2015,5:15561
Murphy, AM, Wong, AL, Bezuhly M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair, 2015,8:7
Zielonka, TM, Zycinska, K, Chorostowska- Wynimko, J, et al. Angiogenic activity of sera from interstitial lung disease patients in relation to angiotensin-converting enzyme activity. Adv Exp Med Biol, 2013,756:213–221
Xu, H, Yang, F, Sun, Y, et al. A new antifibrotic target of Ac-SDKP: inhibition of myofibroblast differentiation in rat lung with silicosis. PLoS One, 2012,7(7):e40301
Fraga-Silva, RA, Ferreira, AJ, Dos Santos, RA. Opportunities for targeting the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor pathway in hypertension. Curr Hypertens Rep, 2013,15(1):31–38
Meng, Y, Yu, CH, Li, W, et al. Angiotensin-Converting Enzyme 2/Angiotensin- (1-7)/Mas Axis Protects against Lung Fibrosis by Inhibiting the MAPK/NF-κB Pathway. Am J Respir Cell Mol Biol, 2014,50(4):723–736
Liu, Y, Xu, H, Geng, YC, et al. Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Resp Res, 2017,18:38
Hemmati, AA, Nazari, Z, Samei M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther, 2008,21(4):668–674
Wang, Y, Yang, GX, Zhu, ZH, et al. Effect of bone morphogenic protein-7 on the expression of epithelial-mesenchymal transition markers in silicosis model. Exp Mol Pathol, 2015,98(3):393–402
Zhou, B, Liu, Y, Kahn, M, et al. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem, 2012,287(3):7026–7038
Tumelty, KE, Smith, BD, Nugent, MA, et al. Aortic carboxypeptidase-like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor beta receptor-dependent and -independent pathways. J Biol Chem, 2014,289(5):2526–2536
Perret-Guillaume, C, Joly, L, Jankowski, P, et al. Benefits of the RAS blockade: clinical evidence before the ONTARGET study. J Hypertens Suppl, 2009,27(2):S3–7
Pereira, RM, dos Santos, RA, da Costa Dias, FL, et al. Renin-angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol, 2009,15(21):2579–2586
Tharaux, PL, Chatziantoniou, C, Fakhouri, F, et al. Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension, 2000,36(3):330–336
Yaghini, FA, Song, CY, Lavrentyev, EN, et al. Angiotensin II -induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension, 2010,55(6):1461–1467
Xie, Z, Singh, M, Singh K. ERK1/2 and JNKs, but not p38 kinase, are involved in reactive oxygen species-mediated induction of osteopontin gene expression by angiotensin II and interleukin-1beta in adult rat cardiac fibroblasts. J Cell Physiol, 2004,198(3):399–407
Specks, U, Martin WJ 2nd, Rohrbach, MS. Bronchoalveolar lavage fluid angiotensin-converting enzyme in interstitial lung diseases. Am Rev Respir Dis, 1990,141(1):117–123
Li, X, Molina-Molina, M, Abdul-Hafez, A, et al. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 2006,291(5):887–895
Grönhagen-Riska, C, Kurppa, K, Fyhrquist, F, et al. Angiotensin-converting enzyme and lysozyme in silicosis and asbestosis. Scand J Respir Dis, 1978,59(4):228–231
Nordman, H, Koskinen, H, Fröseth B. Increased activity of serum angiotensin-converting enzyme in progressive silicosis. Chest, 1984,86(2):203–207
Zelko, IN, Jianxin, Z, Ritzenthaler, JD, et al. Pulmonary hypertension and vascular remodeling in mice exposed to crystalline silica. Respir Res, 2016,17(1):160
Xiaojun, W, Yan, L, Hong, X, et al. Acetylated α-Tubulin Regulated by N-Acetyl-Seryl-Aspartyl-Lysyl-Proline (Ac-SDKP) Exerts the Anti-fibrotic Effect in Rat Lung Fibrosis Induced by Silica. Sci Rep, 2016,6:32257
Uhal, BD, Li, X, Piasecki, CC, et al. Angiotensin signaling in pulmonary fibrosis. Int J Biochem Cell Biol, 2012,44(3):465–468
Tipnis, SR, Hooper, NM, Hyde, R, et al. A human homolog of angiotensin-converting enzyme, cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem, 2000,275(43):33238–33243
Santos, RA, Ferreira, AJ, Verano-Braga, et al. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol, 2013,216(2):R1–R17
Simões E, Silva, AC, Teixeira, MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res, 2016,107:154–162
Chappell M C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol, 2016,310(2):H137–H152
Miyajima, A, Kosaka, T, Kikuchi, E, et al. Renin-angiotensin system blockade: Its contribution and controversy. Int J Urol, 2015,22(8):721–730
Li, X, Molina-Molina, M, Abdul-Hafe A. Angintensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol, 2008,295(1):178–185
Wösten-van Asperen, RM, Lutter, R, Specht, PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol, 2011,225(4):618–627
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 81472953), Natural Science Foundation of Hebei Province (No. H2016209170), Graduate Student Innovation Fund of Hebei Province (No. 2016196), Graduate Student Innovation Fund of North China University of Science and Technology (No. 2016B10) and Undergraduate Innovative Project of North China University of Science and Technology (No. X2017354).
Conflict of Interest Statement
We declare that there are no relevant financial or non-financial conflicts of interest in the study.
Rights and permissions
About this article
Cite this article
Zhang, Bn., Zhang, X., Xu, H. et al. Dynamic Variation of RAS on Silicotic Fibrosis Pathogenesis in Rats. CURR MED SCI 39, 551–559 (2019). https://doi.org/10.1007/s11596-019-2073-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-019-2073-8