Summary
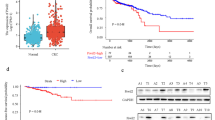
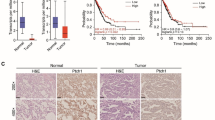
Pituitary adenomas (PAs) are well known as a common intracranial benign tumor, and a portion of PAs are refractory to current therapeutic methods. ErbB receptors family signaling pathway regulates the expression of PAs activation associated gene. Inhibition of epidermal growth factor receptor (EGFR) can inhibit proliferation of PAs. Leucine-rich repeats and immunoglobulin-like domains protein 1 ( LRIG1), a negative mediated gene of ErbB receptors family, plays a role in many tumors. However, there are seldom researches about the functional role of LRIG1 in PAs. The aim of this study is to explore the potential effect of LRIG1 and its regulating mechanism in PAs. First, we investigated the role of LRIG1 in cell migration, invasion of PAs with transfected LRIG1 or control. Then, we explored its impact on cell proliferation and apoptosis of PAs in vivo. To study the regulating mechanism of LRIG1, we examined the expression of molecular factor of PI3K/AKT and Ras/Raf/ERK pathway using Western blotting in vitro and RT-PCR in vitro and in vivo. It was found that LRIG1 over-expression inhibited cell migration, invasion and proliferation, and promoted apoptosis of PAs in vivo and in vitro. Furthermore, LRIG1 suppressed the expression of signaling of PI3K/AKT and Ras/Raf/ERK pathways in PAs. LRIG1, as a negative mediated gene of tumor, can inhibit biological function of PAs via inhibiting PI3K/AKT and Ras/Raf/ERK pathways, and it might be a new target for gene therapy of PAs.
Similar content being viewed by others
References
Di Ieva A, Rotondo F, Syro L V, et al. Aggressive pituitary adenomas—diagnosis and emerging treatments. Nat Rev Endocrinol, 2014,10(7):423–435
Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest, 2003,112(11):1603–1618
Gillam MP, Molitch ME, Lombardi G, et al. Advances in the treatment of prolactinomas. Endocr Rev, 2006,27(5):485–534
Newell-Price J, Bertagna X, Grossman A B, et al. Cushing's syndrome. Lancet, 2006,367(9522):1605–1617
Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell, 2001,104(5):699–708
Andersson U, Guo D, Malmer B, et al. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol, 2004,108(2):135–142
Fukuoka H, Cooper O, Ben-Shlomo A, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest, 2011,121(12):4712–4721
Onguru O, Scheithauer BW, Kovacs K, et al. Analysis of epidermal growth factor receptor and activated epidermal growth factor receptor expression in pituitary adenomas and carcinomas. Mod Pathol, 2004,17(7):772–780
Theodoropoulou M, Arzberger T, Gruebler Y, et al. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J Endocrinol, 2004,183(2):385–394
Vlotides G, Siegel E, Donangelo I, et al. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res, 2008,68(15):6377–6386
Fukuoka H, Cooper O, Mizutani J, et al. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol, 2011,25(1):92–103
Guo D, Holmlund C, Henriksson R, et al. The LRIG gene family has three vertebrate paralogs widely expressed in human and mouse tissues and a homolog in Ascidiacea. Genomics, 2004,84(1):157–165
Hedman H, Nilsson J, Guo D, et al. Is LRIG1 a tumour suppressor gene at chromosome 3p14.3? Acta Oncol, 2002,41(4):352–354
Guo D, Han L, Shu K, et al. Down-regulation of leucine-rich repeats and immunoglobulin-like domain proteins (LRIG1-3) in HP75 pituitary adenoma cell line. J Huazhong Univ Sci Technolog Med Sci, 2007,27(1):91–94
Ye F, Gao Q, Xu T, et al. Upregulation of LRIG1 suppresses malignant glioma cell growth by attenuating EGFR activity. J Neurooncol, 2009,94(2):183–194
Zhang H, Yan Q, Xu S, et al. Association of expression of Leucine-rich repeats and immunoglobulin-like domains 2 gene with invasiveness of pituitary adenoma. J Huazhong Univ Sci Technolog Med Sci, 2011,31(4):520–523
Laederich M B, Funes-Duran M, Yen L, et al. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J Biol Chem, 2004,279(45):47050–47056
Gur G, Rubin C, Katz M, et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J, 2004,23(16):3270–3281
Stutz MA, Shattuck DL, Laederich MB, et al. LRIG1 negatively regulates the oncogenic EGF receptor mutant EGFRvIII. Oncogene, 2008,27(43):5741–5752
Yokdang N, Hatakeyama J, Wald J H, et al. LRIG1 opposes epithelial-to-mesenchymal transition and inhibits invasion of basal-like breast cancer cells. Oncogene, 2016,35:1932–2947
Sheu JJ, Lee CC, Hua CH, et al. LRIG1 modulates aggressiveness of head and neck cancers by regulating EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling. Oncogene, 2014,33(11):1375–1384
Liu X, Kano M, Araki T, et al. ErbB receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology, 2015,156(1):71–79
Cooper O, Mamelak A, Bannykh S, et al. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine, 2014,46(2):318–327
Zhang X, Song Q, Wei C, et al. LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell Stress Chaperones, 2015,20(4):631–641
Kou C, Zhou T, Han X, et al. LRIG1, a 3p tumor suppressor, represses EGFR signaling and is a novel epigenetic silenced gene in colorectal cancer. Biochem Biophys Res Commun, 2015,464(2):519–525
Yang JA, Liu BH, Shao LM, et al. LRIG1 enhances the radiosensitivity of radioresistant human glioblastoma U251 cells via attenuation of the EGFR/Akt signaling pathway. Int J Clin Exp Pathol, 2015,8(4):3580–3590
Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov, 2006,5(8):671–688
Zhou J, Zhi X, Wang L, et al. Linc00152 promotes proliferation in gastric cancer through the EGFR-dependent pathway. J Exp Clin Cancer Res, 2015,34(1):135
Liu JF, Tsao YT, Hou CH. Amphiregulin enhances intercellular adhesion molecule-1 expression and promotes tumor metastasis in human osteosarcoma. Oncotarget, 2015,6(38):40880–40895
Yang Y, Sheng M, Huang F, et al. Downregulation of insulin-like growth factor binding protein 6 is associated with ACTH-secreting pituitary adenoma growth. Pituitary, 2014,17(6):505–513
Bruhn MA, Pearson RB, Hannan RD, et al. Second AKT: the rise of SGK in cancer signaling. Growth Factors, 2010,28(6):394–408
Gao J, Liu X, Yang F, et al. By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM inhibits breast cancer cell growth and metastasis. Oncotarget, 2015,6(29):27187–27198
Hu Y, Liu HX, He Y, et al. Transcriptome profiling and genome-wide DNA binding define the differential role of fenretinide and all-trans RA in regulating the death and survival of human hepatocellular carcinoma Huh7 cells. Biochem Pharmacol, 2013,85(7):1007–1017
Han T, Xiang DM, Sun W, et al. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol, 2015,63(3):651–660
Author information
Authors and Affiliations
Corresponding authors
Additional information
The authors contributed equally to this work.
This project was supported by grants from the National Natural Science Foundation of China (No. 81560412), Jiangxi Provincial Health Development Planning Commission Project (No. 20141065), and Jiangxi Provincial Natural Science Foundation of China (No. 20152BCB24009 and No. 20151BDH80009).
Rights and permissions
About this article
Cite this article
Cheng, Sq., Fan, Hy., Xu, X. et al. Over-expression of LRIG1 suppresses biological function of pituitary adenoma via attenuation of PI3K/AKT and Ras/Raf/ERK pathways in vivo and in vitro . J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 558–563 (2016). https://doi.org/10.1007/s11596-016-1625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1625-4