Abstract
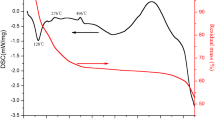
Al2O3-ZrO2 microspheres were prepared by internal gelation method. The effects of Al3+ on the stability of solution and performance of gel spheres were studied. Al3+ had a great influence on the stability of the solutions, and the more of the amount of Al3+, the shorter of the stabilization time. Because Al3+ did not copolymerize with Zr4+ during the sol-gel transformation, the strength of gel sphere added with Al3+ was low and deformed easily as it was squeezed. The results of our experiments well verify Glasser team’s speculation and conclusions. At the same time, based on the experimental results, we prepared Al2O3-ZrO2 composite microspheres with higher content of Al2O3 by controlling the pH of the solution. The change curve of viscosity with time and the stabilization time of the solution with different Al3+ dosage were given, which could provide references for industrial mass production. Samples without hydrothermal treatment cracked severely, while the samples hydrothermally treated kept structural integrity with no cracks after calcined. Al2O3-ZrO2 microspheres with no segregation and phase separation were prepared and alumina evenly distributed in the zirconia matrix. When the content of Al2O3 was low, the tetragonal phase was stable. And the cubic phase was obtained when the content of Al2O3 was more.
Similar content being viewed by others
References
Leray S. Nuclear Waste Transmutation[J]. Nuclear Instruments and Methods in Physics Research, 1996, 113(1–4): 495–500
Bowman C D, Arthur E D, Lisowski P W, et al. Nuclear Energy Generation and Waste Transmutation Using An Accelerator-driven Intense Thermal Neutrons Source[J]. Nuclear Instruments and Methods in Physics Research A, 1992, 320:336–341
Voronkov V A, Dyachenko V M, Kostin V. Neutron Generation in Extended Lead Targets Using the Synchrophasotron Beams[J]. Atomn Energia, 1990, 68: 449–453
Liang T X, Tang C H. Transmutation of Long-lived Nuclides[J]. Nuclear Techniques, 2003, 12(26): 935–939
Liang T X, Guo W L, Hao S C, et al. Inert Matrix Kernels for Pu Incineration in HTR[J]. Nuclear Power Engineering, 2007, 28(SI): 1–3
Abu-Khader MM. Recent Advances in Nuclear Power: A Review[J]. Prog. Nucl. Eng., 2009, 51: 225–235
Hench L L, Clark D E, Campbell J. High Level Waste Immobilization Forms[J]. Nucl. Chem. Waste Manag., 1984, 5: 149–173
Degueldre C, Paratte J M. Concepts for An Inert Matrix Fuel: An Overview[J]. J. Nucl. Mater., 1999, 274: 1–6
International Atomic Energy Agency. Viability of Inert Matrix Fuel in Reducing Plutonium Amounts in Reactors[S]. IAEA-TECDOC-1516, 2006
Chauvin N, Konings RJM, Matzke H. Optimization of Inert Matrix Fuel Concepts for Americium Transmutation[J]. J. Nucl. Mater., 1999, 274: 105–111
Fernandez A, Konings RJM, Somers J. Design and Fabrication of Specific Ceramic-metallic Fuels and Targets[J]. J. Nucl. Mater., 2003, 319: 44–50
Tatsumi A, Kazuya I, Kazuhiro Y, et al. Application of Internal Gelation to Sol-gel Synthesis of Ceria-doped Zirconia Microspheres as Nuclear Fuel Analogous Materials[J]. Journal of Alloys and Compounds, 2005, 394: 271–276
Idemitsu K, Arima T, Inagaki Y, et al. Manufacturing of Zirconia Microspheres Doped with Erbia, Yttria and Ceria by Internal Gelation Process as A Part of A Cermet Fuel[J]. Journal of Nuclear Materials, 2003, 319: 31–36
Oversby V M, McPheeters C C, Degueldre C, et al. Control of Civilian Plutonium Inventories Using Burning in A Non-Fertile Fuel[J]. J. Nucl. Mater., 1997, 245: 17–26
Maschek W, Chen X, Delage F, et al. Accelerator Driven Systems for Transmutation: Fuel Development, Design and Safety[J]. Progress in Nuclear Energy, 2008, 50: 333–340
Wang L, Liang T X. Ceramics for High Level Radioactive Waste Solidification[J]. Journal of Advanced Ceramics, 2012, 1(3): 194–203
Fernandez A, Haas D, Hiernaut J P, et al. Overview of ITU Work on Inert Matrix Fuels[R]. In 9th IEMP, 2006
Fu X M, Liang T X, Tang Y P, et al. Preparation of UO2 Kernel for HTR-10 Fuel Element[J]. Journal of Nuclear Science and Technology, 2004, 41: 942–948
Guo W L, Liang T X. Modeling of Air Region Height in the Casting Process for Preparing UO2 Kernels[J]. Nuclear Engineering and Design, 2011, 241: 2780–2782
Zhou X W, Ma J T, Hao S C, et al. Preparation of Ammonium Diuranate Particles by External Gelation Process of Uranium in INET[J]. Nuclear Engineering and Design, 2012, 250: 192–196
Haas P A. Formation of Uniform Liquid Drops by Application of Vibration to Laminar Jets[J]. Industrial & Engineering Chemistry Research, 1992, 31(3): 959–967
Nazar L F, Klein L C. Early Stages of Alumina Sol-gel Formation in Acidic Media: An 27Al Nuclear Magnetic Resonance Spectroscopy Investigation[J]. J. Am. Ceram. Soc., 1988, 71(2): C–85–C–87
Livage J, Henry M, Sanchez C. Sol-gel Chemistry of Transition Metal Oxides[J]. Prog. Solid St. Chem., 1988, 18:259–341
Zhang W B, Glasser F P. Condensation and Gelation of Inorganic ZrO2-Al2O3 Sols[J]. Journal of Materials Science, 1993, 28: 1129–1135
Zhang W B, Glasser F P. The Preparation of Al2O3- ZrO2 Sol-gels From Inorganic Precursors[J]. Journal of European Ceramic Society, 1993, 11: 143–147
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by National Natural Science Foundation of China (No. 91326203)
Rights and permissions
About this article
Cite this article
Guo, T., Wang, C., Dong, L. et al. Effect of Al2O3 on the Process Performance of ZrO2 Microspheres. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 35, 841–846 (2020). https://doi.org/10.1007/s11595-020-2328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-020-2328-z