Abstract
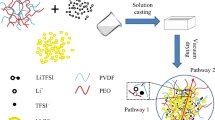
Composite solid electrolyte (CSE), especially the composite room temperature solid electrolyte (CRTSE), is emerging as the promising electrolyte for all-solid-state lithium batteries (ASSLB) due to their ability to combine the desirable properties of ceramic and polymer-based electrolytes and the room temperature operation condition. In this paper, the CRTSE with polyethylene oxide (PEO), bis(fluorosulfonyl)imide (LiTFSI), succinonitrile (SN), LLZTO inorganic fillers, and cross-linked ethoxylated trimethylolpropane triacrylate (ETPTA) was proposed. With the help of lithium dendrite suppression via cross-linked microscopic pore structure, enhancement of the ionic conductivity via LLZTO fillers, and wide electrochemical window via SN, the obtained LCSE showed high ionic conductivity (2.12 × 10−4 S cm−1), high Li+ transfer number (tLi+ = 0.55), and stable electrochemical window (5.0 V vs Li/Li+) at room temperature. The Li symmetrical cell with LCSE can cycle over 500 h stably with current density of 0.1 mA cm−2 and 0.5 mA cm−2 at room temperature. The full solid-state LiFePO4 cell can successfully work over 200 cycles with capacity retention ratio of about 70% at room temperature.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Liu FQ, Wang WP, Yin YX, Zhang SF, Shi JL, Wang L et al (2018) Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci Adv 4:5383. https://doi.org/10.1126/sciadv.aat5383
Li M, Lu J, Chen Z, Amine K (2018) 30 Years of lithium-ion batteries. Adv Mater 30:1800561. https://doi.org/10.1002/adma.201800561
Hu X, Wang R, Feng W, Xu C, Wei Z (2023) Electrocatalytic oxygen evolution activities of metal chalcogenides and phosphides: fundamentals, origins, and future strategies. J Energy Chem 81:167–191. https://doi.org/10.1016/j.jechem.2023.01.062
Chen Y, Wang Z, Li X, Yao X, Wang C, Li Y et al (2020) Li metal deposition and stripping in a solid-state battery via Coble creep. Nature 578:251–255. https://doi.org/10.1038/s41586-020-1972-y
Wang H, Gao H, Chen X, Zhu J, Li W, Gong Z et al (2021) Linking the defects to the formation and growth of Li dendrite in all-solid-state batteries. Adv Energy Mater 11:2102148. https://doi.org/10.1002/aenm.202102148
Yang F, Liu Y, Liu T, Wang Y, Nai J, Lin Z et al (2023) Fluorinated strategies among all-solid-state lithium metal batteries from microperspective. Small Struct 4:2200122. https://doi.org/10.1002/sstr.202200122
Zhao S, Wu Q, Ma W, Yang L (2020) Polyethylene oxide-based composites as solid-state polymer electrolytes for lithium metal batteries: a mini review. Front Chem 8:640. https://doi.org/10.3389/fchem.2020.00640
Jie J, Liu Y, Cong L, Zhang B, Lu W, Zhang X et al (2020) High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J Energy Chem 49:80–88. https://doi.org/10.1016/j.jechem.2020.01.019
Mahant YP, Kondawar SB, Nandanwar DV, Koinkar P (2018) Poly(methyl methacrylate) reinforced poly(vinylidene fluoride) composites electrospun nanofibrous polymer electrolytes as potential separator for lithium ion batteries. Mater Renew Sustain Energy 7:5. https://doi.org/10.1007/s40243-018-0115-y
Zhai W, Zhu H-j, Wang L, Liu X-m, Yang H (2014) Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BF4 for Lithium ion batteries. Electrochim Acta 133:623–630. https://doi.org/10.1016/j.electacta.2014.04.076
Zheng X, Wu J, Wang X, Yang Z (2022) Cellulose-reinforced poly(cyclocarbonate-ether)-based composite polymer electrolyte and facile gel interfacial modification for solid-state lithium-ion batteries. Chem Eng J 446:137194. https://doi.org/10.1016/j.cej.2022.137194
Lu J, Jaumaux P, Wang T, Wang C, Wang G (2021) Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J Mater Chem A 9:24175–24194. https://doi.org/10.1039/D1TA06606D
Choi H, Kim M, Lee H, Jung S, Lee Y-G, Lee YM et al (2022) Unexpected pressure effects on sulfide-based polymer-in-ceramic solid electrolytes for all-solid-state batteries. Nano Energy 102:107679. https://doi.org/10.1016/j.nanoen.2022.107679
Jin Y, Zong X, Zhang X, Jia Z, Xie H, Xiong Y (2022) Constructing 3D Li+-percolated transport network in composite polymer electrolytes for rechargeable quasi-solid-state lithium batteries. Energy Stor Mater 49:433–444. https://doi.org/10.1016/j.ensm.2022.04.035
Li J, Jing M, x, Li R, Li L X, Huang Z H, Yang H, et al (2022) Al2O3 fiber-reinforced polymer solid electrolyte films with excellent lithium-ion transport properties for high-voltage solid-state lithium batteries. ACS Appl Polym Mater 4:7144–7151. https://doi.org/10.1021/acsapm.2c01034
Hua S, Li JL, Jing MX, Chen F, Ju BW, Tu FY et al (2020) Effects of surface lithiated TiO2 nanorods on room-temperature properties of polymer solid electrolytes. Int J Energy Res 44:6452–6462. https://doi.org/10.1002/er.5379
Xu J (2022) Critical Review on cathode–electrolyte Interphase toward high-voltage cathodes for Li-Ion batteries. Nanomicro Lett 14:166. https://doi.org/10.1007/s40820-022-00917-2
Liu S, Liu W, Ba D, Zhao Y, Ye Y, Li Y et al (2023) Filler-integrated composite polymer electrolyte for solid-state lithium batteries. Adv Mater 35:2110423. https://doi.org/10.1002/adma.202110423
Su S, Ma J, Zhao L, Lin K, Li Q, Lv S et al (2021) Progress and perspective of the cathode/electrolyte interface construction in all-solid-state lithium batteries. Carbon Energy 3:866–894. https://doi.org/10.1002/cey2.129
Guo C, Shen Y, Mao P, Liao K, Du M, Ran R et al (2023) Grafting of lithiophilic and electron-blocking interlayer for garnet-based solid-state li metal batteries via one-step anhydrous poly-phosphoric acid post-treatment. Adv Funct Mater 33:2213443. https://doi.org/10.1002/adfm.202213443
Ren Z, Li J, Gong Y, Shi C, Liang J, Li Y et al (2022) Insight into the integration way of ceramic solid-state electrolyte fillers in the composite electrolyte for high performance solid-state lithium metal battery. Energy Stor Mater 51:130–138. https://doi.org/10.1016/j.ensm.2022.06.037
Ha HJ, Kwon YH, Kim JY, Lee SY (2011) A self-standing, UV-cured polymer networks-reinforced plastic crystal composite electrolyte for a lithium-ion battery. Electrochim Acta 57:40–45. https://doi.org/10.1016/j.electacta.2011.03.101
Pervez SA, Kim G, Vinayan BP, Cambaz MA, Kuenzel M, Hekmatfar M et al (2020) Overcoming the interfacial limitations imposed by the solid–solid interface in solid-state batteries using ionic liquid-based interlayers. Nanomicro small 16:2000279. https://doi.org/10.1002/smll.202000279
Ye T, Li L, Zhang Y (2020) Recent progress in solid electrolytes for energy storage devices. Adv Funct Mater 30:2000077. https://doi.org/10.1002/adfm.202000077
Zhang X, Fu C, Cheng S, Zhang C, Zhang L, Jiang M et al (2023) Novel PEO-based composite electrolyte for low-temperature all-solid-state lithium metal batteries enabled by interfacial cation-assistance. Energy Stor Mater 56:121–131. https://doi.org/10.1016/j.ensm.2022.12.048
Sharma JP, Yamada K, Sekhon SS (2012) Conductivity studies of plasticized PEO–HPF6–fumed silica nanocomposite polymer electrolyte system. Ionics 18:151–158. https://doi.org/10.1007/s11581-011-0610-y
Kim SH, Choi KH, Cho SJ, Park JS, Cho KY, Lee CK et al (2014) A shape-deformable and thermally stable solid-state electrolyte based on a plastic crystal composite polymer electrolyte for flexible/safer lithium-ion batteries. J Mater Chem A 2:10854–10861. https://doi.org/10.1039/C4TA00494A
Chen B, Huang Z, Chen X, Zhao Y, Xu Q, Long P et al (2016) A new composite solid electrolyte PEO/Li10GeP2S12/SN for all-solid-state lithium battery. Electrochim Acta 210:905–914. https://doi.org/10.1016/j.electacta.2016.06.025
Rey I, Lassègues JC, Grondin J, Servant L (1998) Infrared and Raman study of the PEO-LiTFSI polymer electrolyte. Electrochim Acta 43:1505–1510. https://doi.org/10.1016/S0013-4686(97)10092-5
Li S, Zhang SQ, Shen L, Liu Q, Ma JB, Lv W et al (2020) Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv Sci 7:1903088. https://doi.org/10.1002/advs.201903088
Xie J, Wang J, Lee HR, Yan K, Li Y, Shi F et al (2018) Engineering stable interfaces for three-dimensional lithium metal anodes. Sci Adv 4:eaat5168. https://doi.org/10.1126/sciadv.aat5168
Gregorio V, García N, Tiemblo P (2022) Ionic conductivity enhancement in UHMW PEO gel electrolytes based on room-temperature ionic liquids and deep eutectic solvents. ACS Appl Polym Mater 4:2860–2870. https://doi.org/10.1021/acsapm.2c00104
Baril D, Michot C, Armand M (1997) Electrochemistry of liquids vs. solids: polymer electrolytes. Solid State Ion 94:35–47. https://doi.org/10.1016/S0167-2738(96)00614-5
Armand M (1994) The history of polymer electrolytes. Solid State Ion 69:309–319. https://doi.org/10.1016/0167-2738(94)90419-7
Li Z, Huang HM, Zhu JK, Wu JF, Yang H, Wei L et al (2019) Ionic conduction in composite polymer electrolytes: case of PEO:Ga-LLZO composites. ACS Appl Mater Interfaces 11:784–791. https://doi.org/10.1021/acsami.8b17279
Zhang J, Zhao N, Zhang M, Li Y, Chu PK, Guo X et al (2016) Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries. Nano Energy 28:447–454. https://doi.org/10.1016/j.nanoen.2016.09.002
Funding
This work was supported by Chongqing Science and Technology Bureau (cstc2021jcyj-msxmX0923), the Organization Department of Chongqing Municipal Committee (Chongqing Talent Innovation and Entrepreneurship Demonstration Team, CQYC20220309959), and Chongqing Education Commission of China (No. KJQN202304407).
Chongqing Municipal Science and Technology Bureau,cstc2021jcyj-msxmX0923,Chongqing Education Commission of China,KJQN202304407,KJQN202304407,Chongqing Talent innovation and entrepreneurship demonstration team,CQYC20220309959
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Bangxing Li, Xianlin Yi, Zhenjun Xie, Fei Wu, Xing Kang, Shuai Kang, and Xiaolin Hu. The first draft of the manuscript was written by Bangxing Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, B., Yi, X., Xie, Z. et al. A promising composite room temperature solid electrolyte via incorporating LLZTO into cross-linked ETPTA/PEO/SN matrix for all solid state lithium batteries. Ionics 30, 2007–2017 (2024). https://doi.org/10.1007/s11581-024-05451-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05451-2