Abstract
Recent times have witnessed notable progress in augmenting the effectiveness of pharmaceutical actions, leading to the creation of novel drug formulations and delivery technologies. A complete understanding of the molecular-level interactions between drug molecules and biological membranes is necessary to achieve optimal design in these processes. Comprehensive understanding of these interactions can be gained through thermodynamic research, which helps pharmaceutical professionals make well-informed decisions about which manufacturing compounds are most suited for a certain application. Because ionic liquids can interact with biological membranes and exert their effects on them, studying ionic liquids in combination with co-solvents in aqueous settings is important for many kinds of research. Using an Anton Paar DSA 5000 M apparatus, the densities, and speed of sound in a liquid mixture comprising L-phenylalanine and glycyl-L-phenylalanine within an aqueous 1-decyl-3-methylimidazolium bromide ([C10mim]Br), the ionic solution was measured. This was done across temperature ranges of 288.15 K, 298.15 K, 308.15 K, and 318.15 K and experimental pressure of p = 0.1 MPa with concentrations of “0.000, 0.005, 0.030, and 0.050 mol kg–1”. From the experimental results, various acoustic and physicochemical properties were derived, including apparent molar properties, partial compression, isentropic compression, and transfer properties. These computations provided insights into intermolecular interactions within the combination of 1-decyl-3-methylimidazolium bromide, water, L-phenylalanine, and glycyl-L-phenylalanine. The mixture’s characteristics were explored through pair and triplet coefficients, taking into account empirical constants and expansibilities, thereby delving into solute–solvent, hydrophilic-hydrophilic, dipole–dipole, and ion-hydrophilic interactions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are liquid substances that maintain their state at or near room temperature and typically consist of organic cations and inorganic anions [1, 2]. ILs offer several advantages, including non-volatility [3,4,5], robust thermal stability [6], a broad chemical range [7], high conductivity [8,9,10], and effective solubility for both inorganic and organic materials [11, 12]. These remarkable attributes make ILs highly versatile and find applications in various fields. For instance, they are employed as electrolyte components for batteries in the electrochemical sector [13,14,15,16,17], serve as efficient reaction environments in engineering processes [18,19,20], and have been explored in pharmaceutical and medical contexts [21]. Moreover, the tunability of ILs, achieved by combining different cations and anions, offers an eco-friendly alternative to conventional solvents. This tunability allows the creation of tailor-made compounds with optimal characteristics for specific applications, thus contributing to environmentally conscious solutions [22,23,24]. In addition to their fundamental significance, investigations into the combination of ionic liquids with water hold a growing practical importance. This is due to the ability to finely adjust the characteristics of ionic liquids not solely through modifications to their molecular structure, but also their amalgamation with a compatible molecular solvent. This phenomenon is referred to as the “fourth evolution” of ionic liquids [25]. Imidazolium-based ionic liquids (ILs) consist of a five-membered imidazolium cation with varying lengths of alkyl chains (n(C) = 0, 1, 2, 4, 6, 8, 10, 12, etc.). These ILs range from 1-methylimidazolium chloride ([C0mim]Cl) to 1-dodecyl-3-methylimidazolium chloride ([C12mim]Cl), encompassing two isomers: 1,2-dimethyl- and 1,3-dimethylimidazolium chloride [26]. Properties of these imidazolium-based ILs, combined with different anions, were extensively investigated through various experimental techniques, including conductometry, densimetry, viscometry, dielectric spectroscopy, vapor pressure osmometry, and speed of sound measurements in both water and various organic solvents.
Proteins serve as a diverse category of organic compounds employed by cells and organs to support their functions and growth. They play a crucial role in enzymatic and genetic regulatory processes [27]. The physicochemical interactions occurring between ionic liquids and primary biomolecules such as amino acids, proteins, carbohydrates, and lipids are pivotal factors for comprehending the pharmacodynamics and pharmacokinetics of these substances. These interactions also play a vital role in formulating drugs within the pharmaceutical industry [28, 29]. A comprehensive exploration of the various thermophysical properties of vitamins in water and aqueous environments containing essential biomolecules offers insights into the distinct molecular interactions between co-solutes and the hydrophilic and hydrophobic components of vitamins. This investigation aids in understanding the conformational stability of biomolecules within biological systems [30, 31]. Furthermore, to enhance the effectiveness of drug formulations, it is essential to possess an understanding of their interactions with amino acids. Amino acids possess a biocompatible nature, which could potentially lead to the formation of stable complexes, thus increasing overall stability. Consequently, a thorough thermodynamic analysis of the drug within aqueous mixtures containing amino acids becomes imperative [32].
The intricate characteristics and diverse molecular interactions found in mixtures can be explored through examinations involving physicochemical and thermodynamic methodologies [33, 34]. Thermodynamic properties play a crucial role in comprehending the various ionic, hydrophilic, and hydrophobic interactions within different solution environments. They offer valuable insights into solute–solute and solute–solvent interactions in the solution phase. Two fundamental thermophysical attributes, namely volume and compressibility, facilitate a thorough understanding of the interactions occurring between solute and solvent molecules within mixtures [35, 36]. In our earlier work, we conducted a systematic examination of interactions within aqueous solutions containing three imidazolium-based ionic liquids, spanning from [C6mim]Br to [C12mim]Br to [C10mim]Br [37,38,39,40,41,42,43]. The findings from these systems revealed that volumetric parameters and acoustic parameters are influenced by the length of the alkyl chain in the ionic liquid. Notably, cations with alkyl chain lengths of n(C) = 6, 10, and 12 were identified as structure-forming agents or kosmotropes. The current study endeavors to comprehensively examine a range of thermodynamic and acoustic parameters within the context of L-phenylalanine and glycyl-L-phenylalanine solvation in aqueous 1-decyl-3-methylimidazolium bromide ([C10mim]Br) solutions at different concentrations (0.005, 0.01, 0.03, and 0.05 mol kg–1). This analysis is carried out under atmospheric pressure and varying temperatures. The parameters under consideration encompass partial and apparent volumes and transfer volumes, as well as interaction coefficients (both pair and triplet). These parameters serve to elucidate the diverse interactions occurring within the system, while also shedding light on the influence of the alkyl chain length of amino acids on their solvation behavior.
Instruments and procedure
Utilized materials
The ionic liquid under investigation, namely 1-decyl-3-methylimidazolium bromide ([C10mim]Br), was synthesized through a process involving acetonitrile, hexane, 1-methylimidazole, and 1-bromodecane. In preparation for this experiment, the compounds underwent vacuum drying and were subsequently stored over P2O5 within a desiccator for 48 h. A comprehensive overview of the chemicals utilized in the current study can be found in Table 1.
Preparation of 1-decyl-3-methylimidazolium bromide
The ionic liquid, 1-decyl-3-methylimidazolium bromide ([C10mim]Br), was synthesized via the direct alkylation process of 1-methylimidazole using an excess of 1-bromododecane in acetonitrile. This reaction took place within a round bottom flask under a nitrogen atmosphere at a temperature of 350.15 K over a period of 48 h. The progress of the reaction was monitored through thin-layer chromatography (TLC). The resulting product was then subjected to drying using a rotary evaporator (Heidolph; type, Basis Hei-VAP ML, 50/60 Hz) under high vacuum conditions at approximately 335.15 K for a minimum of 4 h. The resulting IL exhibited hygroscopic properties. To quantify the moisture content within the prepared sample, a highly sensitive volumetric Karl Fischer analysis titrator was employed. The analysis revealed that the moisture content in 1-dodecyl-3-methylimidazolium bromide was below 0.05 mass fraction. The resultant IL achieved a purity level exceeding 0.97 mass fractions. Spectroscopic analysis conducted using 1H NMR (Bruker 400 MHz) confirmed the absence of significant impurities in the ILs and their alignment with descriptions in existing literature [44, 45]. Supplementary materials, including the IL 1H NMR spectra and chemical shift, are illustrated in Figure S1.
Sample preparation
These samples were prepared using freshly obtained water that underwent triple distillation and degassing. The water had a specific conductance of 10–6 S cm−1. The process of sample preparation involved using a Sartorius CPA225D electronic balance to achieve precise measurements. Typically, the molality of the solutions can be estimated to be accurate within a range of 2 × 10−5 mol·kg−1 in most instances.
Measurement of density and the speed of sound
The density and speed of sound were assessed using the Anton Paar DSA 5000 M from Austria. Detailed explanations of calibrations and methodologies were provided in our previous research [38]. The internal Peltier temperature controller of the instrument ensured temperature stability within 1.0 × 10−3 K. The instrument is capable of determining density with a precision of 0.001 kg m−3 and ultrasonic velocity with a precision of 0.01 m s−1. The experimental uncertainties for density and speed of sound measurements were both below 0.5 kg·m−3 and 1.0 m·s−1, respectively.
Volumetric properties
Apparent molar volume
Thermophysical properties such as density and the speed of sound are essential for accurately characterizing liquid systems. These properties offer the advantages of both quick and precise measurement, while also being sensitive enough to detect even trace amounts of impurities. In this study, the focus was on understanding the volumetric properties of specific mixtures. To achieve this, the experimental densities of L-phenylalanine and glycyl-L-phenylalanine were measured within an aqueous solution containing the ionic liquid 1-decyl-3-methylimidazolium bromide ([C10mim]Br). The measurements were taken across a range of concentrations (in mol kg−1) including 0.000, 0.005, 0.01, 0.03, and 0.05 mol kg–1 and at various temperatures (T, in K) such as 288.15, 298.15, 308.15, and 318.15 and experimental pressure of p = 0.1 MPa.
The densities obtained through experimental measurements for all the solutions have been documented in Table S1. Graphical representations in Fig. 1 illustrate the contrasts between the experimentally derived densities of the aqueous ionic liquid mixture (consisting of 1-decyl-3-methylimidazolium bromide and water) and the data from existing literature sources [46, 47]. Analysis of Fig. 1 indicates a resemblance in trends between the literature-reported values and the experimental findings. Figure 2 visually presents the density information obtained from experimental assessments for glycyl-L-phenylalanine, juxtaposed with data extracted from existing sources in the literature [48, 49]. The density evaluations concerning the binary combination (glycyl-L-phenylalanine + water) exhibit a consistent pattern with the densities outlined in the literature (depicting a decline in density with rising temperature, akin to what is depicted in Fig. 2). This alignment implies a degree of comparability between the two datasets. By applying the formula employed in our prior research [38] endeavors, we successfully calculated the volumetric expansion (Vϕ) utilizing data acquired from experimental density measurements.
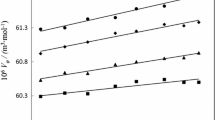
Every symbol retains its conventional significance as documented in our earlier paper. The density and apparent molar volume (Vϕ) data obtained from the experimental work are presented in Table 2. The computed apparent molar volumes are visually represented in Figs. 3 and 4. In Fig. 3, the apparent molar volume of L-phenylalanine is showcased within aqueous solutions containing 1-decyl-3-methylimidazolium bromide, across varying temperatures. Likewise, Fig. 4 portrays the molar volume of glycyl-L-phenylalanine in both aqueous solutions and (1-dodecyl-3-methylimidazolium bromide + water) mixtures, observed at different temperatures. The values recorded for Vϕ in Table S1 exhibit a positive trend, increasing with higher concentrations of 1-decyl-3-methylimidazolium bromide and elevated temperatures. A positive value suggests a pronounced interaction between the solute and solvent.
Partial molar volume
Upon applying a least squares fitting procedure to correlate the apparent molar volume with the equation outlined in our prior publication [38], we derive the partial molar volume (\({V}_{\phi }^{0}\)).
The conventional meanings of every symbol are expounded upon in our earlier paper [38]. The impact of interactions between the solute L-phenylalanine/glycyl-L-phenylalanine and the solvent ionic liquid becomes evident through the asymptotic value of\({V}_{\phi }^{0}\). This value remains constant despite any interactions between the solute and solvent at extremely low concentrations. The slope (\({S}_{V}^{*}\)) observed in the experiment reflects interactions between valine and glycyl-L-valine molecules, serving as a measure of the volumetric pairwise interaction. Table 2 presents the values of both \({V}_{\phi }^{0}\) and\({S}_{V}^{*}\), along with their corresponding standard errors. A clear connection emerges as concentration and temperature exhibit a concurrent rise. Empirical observations illustrate an expanding overlap between the two spheres, resulting in an augmented volume attributed to the coalescing ionic species from diverse co-spheres. Encounters between hydrophobic compounds and ions commonly yield favorable consequences. These interactions work to minimize the overall extent of hydrophobic and hydrophilic contact areas. Elevated temperatures prompt the solvation layer to release a greater number of molecules, thereby amplifying the volume at infinite dilution. With an elevation in solution temperature, there is a minor breakdown of the solvation layer, enabling a slightly increased influx of solvent into the solution and resulting in solution expansion. Notably, as the molar mass of glycyl-L-phenylalanine rises, so does the magnitude of \({V}_{\phi }^{0}\). When compared to temperature, concentrations of whole [C10mim]Br exhibit negative values (\({S}_{V}^{*}\)). Molecules of L-phenylalanine and glycyl-L-phenylalanine exhibit weak interactions within ternary solutions, evidenced by the negative values of \({S}_{V}^{*}\). A smaller \({S}_{V}^{*}\) value leads to the attenuation of solute–solute interactions while intensifying solute–solvent interactions. The presence of a positive \({V}_{\phi }^{0}\) value in solutions of 1-decyl-3-methylimidazolium bromide containing amino acids suggests feeble solute–solute interactions. Given the absence of a consistent trend, it is anticipated that additional factors will modify solute + solute interactions. Larger values indicate a greater prevalence of solute + solvent interactions compared to solute + solute interactions.
Partial molar volume of transfer
The equation employed in our earlier publication [38] was utilized to ascertain the volumes of L-phenylalanine and glycyl-L-phenylalanine that underwent transfer from water to aqueous solutions. This approach also revealed that solute + solvent interactions are more dominant compared to solute + solute interactions when these compounds are dissolved in [C10mim]Br under conditions of infinite dilution.
The parameter \(\Delta {V}_{\phi }^{0}\) offers valuable understanding regarding the interaction between a co-solvent and a solute, excluding any influence from solute–solute interactions. Drawing from our preceding research [41], we successfully determined the concentrations of valine within a water medium. Additionally, in this current study, we computed the partial molar transfer for glycyl-L-phenylalanine. The partial molar volume of transfer values, as calculated, is presented in Table 3. For L-phenylalanine and glycyl-L-phenylalanine, the results are positive values. The presence of positive values suggests robust ion-ion interactions within the ionic liquid containing amino acids. When \(\Delta {V}_{\phi }^{0}\) takes a positive value, it indicates that valine and glycyl-L-phenylalanine may possess the ability to enhance structural arrangements. This positive value aligns with the co-sphere hypothesis, which states that a solute’s potential to form structures corresponds to the strength of interactions among its co-spheres. These co-sphere overlap model parameters not only shed light on interactions between solvents but also provide insight into interactions between solvents themselves.
In accordance with the co-sphere overlap model for ternary mixtures, positive values denote the solute’s capacity to create structures, facilitated by structural interactions between two co-spheres. Notably, when the value is positive, solute + solute interactions tend to be weaker compared to solute + solvent interactions. Potential interactions between a solute and co-solute encompass several scenarios: (1) interactions between polar-ionic groups, (2) interactions between polar-polar groups, (3) interactions between polar-nonpolar groups, and (4) interactions between nonpolar-nonpolar groups. As per this model, (1) and (2) interactions lead to a positive (), whereas (3) and (4) interactions yield a negative \(\Delta {V}_{\phi }^{0}\). The findings of our current investigation underline the significance of polar-ionic group interactions (1) and polar-polar group interactions (2) between the polar groups of valine and glycyl-L-phenylalanine molecules and the polar groups as well as ions within the ionic liquids. These results point toward the heightened importance of these interactions with increasing concentrations of the ionic liquid, indicating their reinforcement within the examined concentration range.
Temperature-dependent properties
We investigated the relationship between temperature and apparent molar volumes at infinite dilution, employing the general polynomial equation as outlined in our previous work [38].
The symbols employed in Eq. (4) retain their conventional meanings, which were elucidated in our previous work [38]. Table 4 presents the constants (a, b, and c) corresponding to L-phenylalanine and glycyl-L-phenylalanine in aqueous solutions of 1-decyl-3-methylimidazole bromide. It is noteworthy that while glycyl-L-phenylalanine has a negative coefficient for c, valine’s coefficient for c is positive. Conversely, both amino acids demonstrate positive values for both coefficients a and b. The variables utilized to compute the theoretical values are also outlined in Table 4. To assess the disparity between theoretical and experimental values, the absolute standard deviation (σ) is calculated using the provided equations and is displayed in Table 4.
In this context, denoted as Y = \({V}_{\phi }^{o}\) s (= Vφ at infinite dilution), it is evident from Table 4 that the polynomial equation effectively provides a close approximation for amino acids and ionic liquids, considering their marginal variations. The absolute temperature serves as a means to express the temperature dependency (T) of the partial molar volume. Utilizing the subsequent formula, the limiting values of apparent molar expansibilities are computed.
Comprehending the interplay of intermolecular forces within the solution [50] is facilitated through the utilization of the limiting apparent molar expansibilities at infinite dilution (\({\phi }_{E}^{0}\)) and (∂\({\phi }_{E}^{0}\)/∂T)p. The system’s aptitude for both creating and dismantling structures finds elucidation in Hepler’s insights [51].
As the concentration of the ionic liquid increases, the resulting data exhibit a somewhat irregular pattern. To decipher these interactions, one can consider the concepts of packing or caging. The positive values of ∂\({\phi }_{E}^{0}\)/∂T for both amino acid and their dipeptide within the system, as presented in Table 5, highlight their capability to foster structural formations. These results originate from two factors \({\phi }_{E}^{0}\) (Elec) and \({\phi }_{E}^{0}\) (Str) which signifies the standard apparent molar expansibility resulting from electrostriction-induced changes (involving the solutes’ surrounding hydration layer), and \({\phi }_{E}^{0}\) (Elec) denotes the standard apparent molar expansibility stemming from shifts in the solvent’s structure. Consequently, when temperatures are lower, \({\phi }_{E}^{0}\) (Str) surpasses \({\phi }_{E}^{0}\) (Elec), while at higher temperatures, \({\phi }_{E}^{0}\) (Elec) supersedes \({\phi }_{E}^{0}\) (Str). The computed values for L-phenylalanine/glycyl-L-phenylalanine within the examined solutions are provided in Table 6, and these figures unveil significant insights about solute–solvent interactions. The favorable outcomes for the tested solutes suggest that solvent molecules might be liberated from solvation layers during heating, showcasing a characteristic of aqueous hydrophobic hydration solutions. These findings underline the presence of contrasting interactions between hydrophilic and hydrophobic molecules. Hepler’s constant, reflecting the sign of the second derivatives of the standard partial molar volume concerning temperature, emerges as a more informative parameter to elucidate a solute’s propensity to form and dismantle structures within solutions.
Acoustical properties
Apparent molar isentropic compression
The apparent molar isentropic compression (Kϕ,s) of the specified systems can be determined using the provided standard equation [38].
The significance of each term within the aforementioned equation is in line with its conventional interpretation, as expounded upon in our previous report [38].
In the provided equation, “c” signifies the experimental speed of sound, and “ρ” represents the solution’s density. Figure 5 draws a comparison between the measured speed of sound within an aqueous ionic liquid mixture (1-decyl-3-methylimidazolium bromide + water) and the data referenced from existing articles [46]. This comparison indicates that while the experimental values align with the literature data in terms of trends at low and high concentrations, there is a noticeable deviation from the literature data. Experimental measurements of the speed of sound for glycyl-L-phenylalanine were matched against literature data [52, 53], leading to the graphical representation displayed in Fig. 6. The speed of sound measurements for the binary mixture (glycyl-L-phenylalanine + water) closely follows the trends reported in the literature. Table S2 presents the experimental speed of sound values and the estimated apparent molar isentropic compression (Kϕ,s) for L-phenylalanine and glycyl-L-phenylalanine. The observations reveal that 1-dodecyl-3-methylimidazolium bromide exhibits a consistently negative apparent molar isentropic compression (Ks) across the entire temperature spectrum. As temperatures rise and 1-decyl-3-methylimidazolium bromide concentrations increase, the unfavorable effects of apparent molar isentropic compression (Ks) become less prominent. Positive outcomes suggest that the aggregation of solute molecules around ionic-charged amino acids has a more substantial influence on the solvent, in comparison to situations resulting in negative outcomes.
Comparison of experimental and literature [46] values of speed of sound for aqueous solution of 1-decyl-3-methylimidazolium bromide + water at different temperatures (a 288.15 K, b 298.15 K, c 308.15 K)
Partial molar isentropic compression
As per the equation employed in our previous paper [38], the apparent molar isentropic compression (Ks) deviates alongside changes in molal concentration.
Here, \({S}_{K}^{*}\) represents the experimental slope that characterizes interactions between (solute–solute), while \({K}_{\phi ,s}^{0}\) stands for the limiting isentropic compression. The molality of amino acids in 1-dodecyl-3-methylimidazolium bromide solution is quantified in moles per liter (mA). The values for the experimental slope (\({S}_{K}^{*}\)), limiting isentropic compression (\({K}_{\phi ,s}^{0}\)), and associated standard errors are presented in Table 6, computed through a least squares fitting approach. Notably, at lower temperatures, substantial interactions between water molecules and amino acids are observed, resulting in negative amino acid values [54]. The negative values signify a weakening of the attractive forces between water molecules and amino acids at higher temperatures, thereby permitting some water molecules to escape into the overall volume of the liquid. In the context of infinite dilution, interactions between solute + solute are minimal, while interactions between solute + solvent hold significance.
Partial molar isentropic compression of transfer
By applying the Eq. (11) extracted from our previous investigation [38], we computed the partial molar compression of amino acids transitioning from water to aqueous solutions containing 1-decyl-3-methylimidazolium bromide.
The corresponding values for L-phenylalanine and glycyl-L-phenylalanine are presented in Table 7. The valine concentrations (\({V}_{\phi }^{0}\)) (in water) were obtained from our preceding investigation [41]. In the current study, we conducted measurements to determine the levels of glycyl-L-phenylalanine in water. For 1-decyl-3-methylimidazolium bromide, the calculated values for both L-phenylalanine and glycyl-L-phenylalanine remain negative across the entire spectrum of temperatures and concentrations. This tabulated data reveals an increase as the concentration of 1-decyl-3-methylimidazolium bromide rises. A low score implies that 1-decyl-3-methylimidazolium bromide ions tend to dominate around an amino acid’s zwitterionic center, indicating their role as structurally developing ions. The interaction between 1-decyl-3-methylimidazolium bromide and zwitterionic amino acid residues strengthens with the escalation of bromide concentrations [55].
Coefficients of interactions
Derived from the McMillan and Mayer hypothesis [56], Friedmann and Krishanan [57] formulated a mathematical approach to approximate the interaction coefficient. Equations (12) and (13) offer a means to calculate the partial molar volume of transfer and partial molar isentropic compression of transfer.
where mB represents the molality of the ionic liquid (1-dodecyl-3-methylimidazolium bromide), A denotes an amino acid, and B corresponds to 1-dodecyl-3-methylimidazolium bromide. The parameters VAB and VABB denote volume-related characteristics, while KAB and KABB signify adiabatic compressibility attributes, collectively used to depict the pair and triplet interaction coefficients. These volumetric and compressibility parameters, VAB, VABB, KAB, and KABB, are harnessed to express the dynamics of pair and triplet interactions. To ascertain these constants, equations incorporating the values of ∆Vϕ0 and \(\Delta {K}_{\phi ,S}^{0}\) were solved, resulting in the determination of pair and triplet interaction coefficients showcased in Table 8. Within the realm of VAB, both positive and negative aspects emerge for L-phenylalanine and glycyl-L-phenylalanine, though values consistently lean toward positivity. Valine’s VABB values manifest a mix of positive and negative outcomes, whereas glycyl-L-phenylalanine predominantly demonstrates beneficial effects. When the VAB evaluation yields affirmative results, amino acids and 1-decyl-3-methylimidazolium bromide interact in pairs. The presence of positive KABB readings indicates the compressibility of both amino acids. Across all temperatures, the double coefficients KAB yield negative values. The contrast between the positive values of VAB and the negative values of VABB for L-phenylalanine and glycyl-L-phenylalanine suggests an overlap in the hydration spheres of solute and co-solute molecules. In amino acid + 1-decyl-3-methylimidazolium bromide + water mixtures, paired interactions are pivotal, as deduced from the volumetric and compressibility analyses, reflecting positive pair interaction coefficients.
Conclusion
In an exploration of the solvation dynamics of L-phenylalanine and glycyl-L-phenylalanine within an aqueous solution of 1-dodecyl-3-methylimidazolium bromide ([C10mim]Br), a comprehensive investigation was conducted on various volumetric and acoustic properties. This encompassed metrics such as apparent molar volumes (Vϕ), apparent molar volumes at infinite dilution (\({V}_{\phi }^{0}\)), partial molar volume of transfer (\(\Delta {V}_{\phi }^{0}\)), partial molar isentropic compression (Kϕ,s), partial molar isentropic compression at infinite dilution (\({K}_{\phi ,s}^{0}\)), and partial molar isentropic compression of transfer (\(\Delta {K}_{\phi ,S}^{0}\)). Spanning temperatures within the range of 288.15 to 318.15 K, the study aimed to elucidate the interactions between solute and solvent. The findings unveiled that the partial molar volume of transfer exhibited positive values, intensifying further with the escalation of 1-dodecyl-3-methylimidazolium bromide concentration. Notably, two prominent interaction types emerged as significant in our investigation: (1) interactions involving polar-ionic groups and (2) interactions between polar groups. Hepler’s constant emerged as a key parameter, providing valuable insights into the potential of amino acids to shape and break down molecular structures.
Data availability
Data will be made available on request.
References
Correia DM, Fernandes LC, Martins PM, GarcíaAstrain C, Costa CM, Reguera J (2020) Ionic liquid–polymer composites: a new platform for multifunctional applications. Adv Funct Mater 30:1909736
Haron GAS, Mahmood H, Noh MH, Alam MZ, Moniruzzaman M (2021) Ionic liquids as a sustainable platform for nanocellulose processing from bioresources: overview and current status. ACS Sustain Chem Eng 9(3):1008–1034
Zhang S, Sun J, Zhang X, Xin J, Miao Q, Wang J (2014) Ionic liquid-based green processes for energy production. Chem Soc Rev 43(22):7838–7869
MacFarlane DR, Meakin P, Sun J, Amini N, Forsyth M (1999) Pyrrolidinium imides: a new family of molten salts and conductive plastic crystal phases. J Phys Chem B 103:4164–4170
Sun J, Forsyth M, MacFarlane DR (1998) Room-temperature molten salts based on the quaternary ammonium ion. J Phys Chem B 102:8858–8864
Mateus NMM, Branco LC, Lourencüo NMT, Afonso CAM (2003) Synthesis and properties of tetra-alkyl-dimethylguanidinium salts as a potential new generation of ionic liquids. Green Chem 5:347–352
Forsyth SA, MacFarlane DR (2003) 1-Alkyl-3-methylbenzotriazolium salts: ionic solvents and electrolytes. J Mater Chem 13:2451–2456
MacFarlane DR, Forsyth SA, Golding J, Deacon GB (2002) Ionic liquids based on imidazolium, ammonium and pyrrolidinium salts of the dicyanamide anion. Green Chem 4:444–448
Pringle JM, Golding J, Forsyth CM, Deacon GB, Forsyth M, MacFarlane DR (2002) Physical trends and structural features in organic salts of the thiocyanate anion. J Mater Chem 12:3475–3480
Golding J, Forsyth S, MacFarlane DR, Forsyth M, Deacon GB (2002) Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: novel low cost ionic liquids. Green Chem. 4:223–229
Yoshida Y, Muroi K, Otsuka A, Saito G, Takahashi M, Yoko T (2004) 1-Ethyl-3-methylimidazolium based ionic liquids containing cyano groups: synthesis, characterization, and crystal structure. Inorg Chem 43:1458–1462
Zhang Y, Zhang X, Lang X, Zhang Q (2021) Study on the themodynamic properties and electrochemical performance of mixture electrolyte for supercapacitor composed of ionic liquid[BMIM][BF4] and SBPBF4/PC. Ionics 27(9):4003–4011
Liu H, Yu H (2019) Ionic liquids for electrochemical energy storage devices applications. J Mater Sci Technol 35(4):674–686
Zhang K, Zhou G, Fang T, Jiang K, Liu X (2021) Structural reorganization of ionic liquid electrolyte by a rapid charge/discharge circle. J Phys Chem Lett 12(9):2273–2278
Sampaio AM, Siqueira LJA (2020) Ether-functionalized sulfonium ionic liquid and its binary mixtures with acetonitrile as electrolyte for electrochemical double layer capacitors: a molecular dynamics study. J Phys Chem B 124:6679–6689
Shama VM, Swami AR, Aniruddha R, Sreedhar I, Reddy BM (2021) Process and engineering aspects of carbon capture by ionic liquids. J CO2 Util 48:101507
Egorova KS, Gordeev EG, Ananikov VP (2017) Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev 117(10):7132–7189
Zeng S, Zhang X, Bai L, Zhang XC, Wang H, Wang JJ, Bao D, Li MD, Liu XY, Zhang SJ (2017) Ionic-liquid-based CO2 capture systems: structure, interaction and process. Chem Rev 117:9625–9673
Ventura SPM, Silva FA, Quental MV, Mondal D, Freire MG, Coutinho JA (2017) Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem Rev 117:69847052
Faria RAM, Vieira TFM, Melo CI, Bogel-Łukasik E (2016) Solubility data as a response for a challenge for formulation chemists: imidazolium-based ionic liquids and antitubercular antibiotic medicines. J Chem Eng Data 61:3116–3126
Wu J, Wu X, Wu R, Wang Z, Tan N (2021) Research for improvement on the extract efficiency of lignans in traditional Chinese medicines by hybrid ionic liquids: as a case of Suhuang antitussive capsule. Ultrason Sonochem 73:105539
Wang Z, Zhang J, Lu B, Li YB, Liang YH, Yuan JM, Zhao M, Wang BS, Mai CK, Zhang JH (2019) Novel bio–renewable matrinium–based ionic liquids derived from Chinese herb medicine: synthesis, physicochemical properties and biological activity. J Mol Liq 296:111822
Triolo A, Russina O, Keiderling U, Kohlbrecher J (2006) Morphology of poly (ethylene oxide) dissolved in a room temperature ionic liquid: a small angle neutron scattering study. J Phys Chem B 110:1513–1515
Bai L, Wang T, Weisensee PB, Liu X, He M (2021) Two–binary–interaction parameter model for molecular solute+ionic liquid solution. Ind Eng Chem Res 60(30):11490–11501
Williams DBG, Ajam M, Ranwell A (2007) High rate and highly selective vinyl acetate hydroformylation in ionic liquids as solvent or cosolvent. Organometallics 26(18):4692–4695
Feder-Kubis J, Gardas RL, Geppert-Rybczyn´ska M (2021) On the influence of the menthol moiety on the transport properties of a homologue series of functionalized bis (trifluoromethylsulfonyl) imide room–temperature ionic liquids: a quest for the structure–property relationship. J Phys Chem B 125:8502–8510
Ayranci G, Sahin M, Ayranci E (2007) Volumetric properties of ascorbic acid (vitamin C) and thiamine hydrochloride (vitamin B1) in dilute HCl and in aqueous NaCl solutions at (283.15, 293.15, 298.15, 303.15, 30815, and 31315)K. J Chem Thermodyn. 39(12):1620–1631
Bhattacharya DM, Kawadkar DV, Pandhurnekar CP, Wankhade AV, Pratap UR, Zodape SP (2017) Investigation of volumetric and acoustic properties of procainamide hydrochloride in aqueous binary and (water+amino acid) ternary mixtures at different temperatures. J Chem Eng Data 62(12):4083–4092
Khatun MR, Islam MM, Rima FR, Islam MN (2016) Apparent molar volume, adiabatic compressibility, and critical micelle concentration of flucloxacillin sodium in aqueous NaCl solutions at different temperatures. J Chem Eng Data 61(1):102–113
Bhattacharya DM, Dhondge SS, Zodape SP (2016) Solvation behaviour of an antihelmintic drug in aqueous solutions of sodium chloride and glucose at different temperatures. J Chem Thermodyn 101:207–220
Singla M, Kumar H, Jindal R (2014) Solvation behaviour of biologically active compounds in aqueous solutions of antibacterial drug amoxicillin at different temperatures. J Chem Thermodyn 76:100–115
Chauhan S, Pathania L, Chauhan M (2016) Thermo-acoustical and optical studies of glycine and dl-alanine in aqueous furosemide solutions at different temperatures. J Mol Liq 221:755–762
Dhondge SS, Zodape SP, Parwate DV (2012) Volumetric and viscometric studies of some drugs in aqueous solutions at different temperatures. J Chem Thermodyn 48:207–212
Pal A, Soni S (2013) Volumetric properties of glycine in aqueous solutions of some sulfa drugs at (288.15, 298.15, and 308.15) K. J Chem Eng Data 58(1):18–23
Singh V, Panda S, Kaur H, Banipal PK, Gardas RL, Banipal TS (2016) Solvation behavior of monosaccharides in aqueous protic ionic liquid solutions: volumetric, calorimetric and NMR spectroscopic studies. Fluid Phase Equilib 421:24–32
Torres DR, Blanco LH, Martínez F, Vargas EF (2007) Apparent molal volumes of lidocaine HCl and procaine HCl in aqueous solution as a function of temperature. J Chem Eng Data 52(5):1700–1703
Kumar H, Sharma R (2020) Influence of 1-hexyl-3-methylimidazolium bromide ionic liquid on the volumetric and acoustic properties of amino acids (L-alanine and L-phenylalanine) at different temperatures. J Mol Liq 304:112666
Kumar H, Sharma R (2021) Solvation properties of glycine and glycylglycine in aqueous 1-hexyl-3-methylimidazolium bromide solution at different temperatures. J Chem Thermodyn 152:106268
Kumar H, Sharma R, Kumar V, Alothman AA (2021) Exploration of the solvation behavior of the synthesized 1-hexyl-3-methylimidazolium bromide [C6mim][Br] ionic liquid with L-cysteine and N-acetyl L-cysteine) in aqueous medium at different temperatures. J Mol Liq 324:114664
Kumar H, Sharma R, Singla M (2021) Studies on volumetric and acoustic behfavior of L-alanine and L-leucine in aqueous 1-dodecyl-3-methylimidazolium bromide ionic liquid solutions at different temperatures. J Mol Liq 342:117022
Sharma R, Kumar H, Singla M, Kumar V, Al-Kahtani AA, Pandey S (2022) Volumetric and acoustic properties of L-phenyl glycine and L-phenylalanine in aqueous solution of 1-dodecyl-3-methylimidazolium bromide [C12mim] [Br]. J Mol Liq 357:119014
Sharma R, Kumar H, Singla M, Kallem P, Banat F (2022) Solvation properties of branched-chain amino acids in aqueous solutions of 1-dodecyl-3-methylimidazolium bromide at different temperatures: volumetric and acoustic measurements. J Chem Eng Data 67:2225–2241
Sharma R, Kumar H, Singla M, Kumar V, Ansar S, Girdhar K (2022) Evolution of solute–solvent interactions for aqueous 1-decyl-3-methylimidazolium bromide, [C10mim] [Br] solutions containing glycine/ glycylglycine through volumetric and acoustic studies. Chem Data Collec 42:100963
Dupont J, Consort CS, Suarez AZ, Souza RF (1999) Preparation of 1-butyl-3-methyl imidazolium-based room temperature ionic liquids. Org Synth 79:236–239
Holbrey JD, Seddon KR (1999) The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J Chem Soc Dalton Trans 2133–2140
Nader O, Sadeghi R (2016) Effect of temperature on the aggregation behaviour and thermodynamic properties of surface active ionic liquid 1-decyl-3-methylimidazolium bromide in aqueous solutions: surface tension, vapour pressure osmometery, conductivity, volumetric and compressibility study. J Chem Thermodyn 102:68–78
Gaillon L, Sirieix-Plenet J, Letellier P (2004) Volumetric study of binary solvent mixtures constituted by amphiphilic ionic liquids at room temperature (1-alkyl-3-methylimidazolium bromide) and water. J Solut Chem 33:11
Reading JF, Hedwig GR (1989) Thermodynamic properties of peptide solutions 4. Partial molar volumes and heat capacities of aqueous solutions of some glycyl dipeptides. J Solut Chem 18:2
Hedwig GR, Hastie JD, Hoiland H (1996) Thermodynamic properties of peptide solutions: 14. Partial molar expansibilities and isothermal compressibilities of some glycyl dipeptides in aqueous solution. J Solut Chem 25:7
Sinha B, Sarkar A, Roy P, Brahman D (2011) Physicochemical properties of L-alanine in aqueous silver sulphate solutions at (298.15, 308.15, and 318.15) K. Int J Thermophys 32:2062–2078
Hepler LG (1969) Thermal expansion and structure in water and aqueous solutions. Can J Chem 47:4613–4617
Hedwig GR, Hoiland H, Hogseth E (1996) Thermodynamic properties of peptide solutions. Part 15. Partial molar isentropic compressibilities of some glycyl dipeptides in aqueous solution at 15 and 350C. J Solut Chem 25:11
Hedwig GR, Hoiland H (1991) Thermodynamic properties of peptide solutions: 7. Partial molar isentropic pressure coefficients of some dipeptides in aqueous solution. J Solut Chem 20:11
Zafarani-Moattar MT, Sarmad S (2010) Effect of tri-potassium phosphate on volumetric, acoustic, and transport behaviour of aqueous solutions of 1-ethyl-3-methylimidazolium bromide at T = (298.15 to 318.15) K. J Chem Thermodyn 42:1213–1221
Romero CM, Negrete F (2004) Effect of temperature on partial molar volumes and viscosities of aqueous solutions of α-dl-aminobutyric acid, dl-norvaline and dl-norleucine. Phys Chem Liq 42:261–267
Friedman HL, Krishnan CV (1973) Enthalpies of alkyl sulfonates in water, heavy water, and water-alcohol mixtures and the interaction of water with methylene groups. J Solut Chem 2:37–51
McMillan WG, Mayer JE (1945) The statistical thermodynamics of multicomponent systems. J Chem Phys 13:276–305
Acknowledgements
The authors are also grateful to Dr. B. R. Ambedkar National Institute of Technology, Jalandhar’s Director and Head, Department of Chemistry, for giving necessary laboratory facilities. The MHRD provided a fellowship to one of the authors (Ravinder Sharma). Also, the KSU author acknowledges the financial support through Researchers Supporting Project number (RSPD2024R688), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by North-West University. The authors are also grateful to Dr. B. R. Ambedkar National Institute of Technology,Jalandhar's Director and Head,Department of Chemistry,for giving necessary laboratory facilities. The MHRD provided a fellowship to one of the authors (Ravinder Sharma). Also, the KSU author acknowledges the financial support through Researchers Supporting Project number (RSPD2024R688), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
R.S.: data curation, formal analysis, visualization, writing—original draft.
I.B.: conceptualization, visualization, review and editing.
M.G.: data curation, resources.
M.M.S.A.: funding acquisition, writing—reviewing and editing.
S.S.: data curation, formal analysis.
K.T.: review and editing.
All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, R., Bahadur, I., Gautam, M. et al. Interaction study of L-phenylalanine/glycyl-L-phenylalanine in water-soluble 1-decyl-3-methylimidazolium bromide ([C10mim]Br) ionic liquid: thermodynamic/physicochemical approaches. Ionics 30, 1653–1667 (2024). https://doi.org/10.1007/s11581-023-05360-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05360-w