Abstract
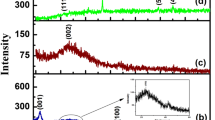
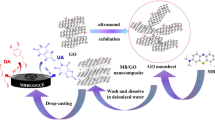
Porous reduced graphene oxide (pr-GO) exhibits good electrical conductivity, higher surface area and higher sensitivity. Herein, we have synthesized pr-GO by solution combustion method and characterized by XRD, FT-IR, SEM, Raman and absorption spectral studies used for the simultaneous determination of dopamine (DA) and uric acid (UA). The pr-GO-modified electrode showed good electrocatalytic activity and separated well the oxidation peaks of DA and UA. A simple and sensitive differential pulse voltammetric method was proposed for the simultaneous determination of DA and UA in the range of 0.5–470.8 µM and 0.1–450.1 µM, respectively, at pr-GO-modified electrode with LOD (s/n = 3) values of 0.031 µM and 0.060 µM for DA and UA, respectively.









Similar content being viewed by others
Data availability
This declaration is not applicable.
References
Tao Y, Lin Y, Ren J, Qu X (2013) A dual fluorometric and colorimetric sensor for dopamine based on BSA-stabilized Au nanoclusters. Biosens Bioelectron 42:41–46
Ma W, Liu HT, Long YT (2015) Monitoring dopamine quinone-induced dopaminergic neurotoxicity using dopamine functionalized quantum dots. ACS Appl Mater Interfaces 7:14352–14358
Carvalho LAC, Lopes JPPB, Kaihami GH et al (2018) Uric acid disrupts hypochlorous acid production and the bactericidal activity of HL-60 cells. Redox Biol 16:179–188
Wang KH, Penmatsa A, Gouaux E (2015) Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521(7552):322–327
Ning J, He Q, Luo X et al (2018) Determination of uric acid in co-presence of dopamine and ascorbic acid using cuprous oxide nanoparticle-functionalized graphene decorated glassy carbon electrode. Catalysts 8:407
Wan X, Yang S, Cai Z et al (2019) Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 9:847
Cai Z, Ye Y, Wan X et al (2019) Morphology–dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials 9:835
Wu Y, Deng P, Tian Y et al (2020) Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J Nanobiotechnology 18:1–13
Wu B, Xiao L, Zhang M et al (2021) Facile synthesis of dendritic-like CeO2/rGO composite and application for detection of uric acid and tryptophan simultaneously. J Solid State Chem 296:122023
Deng P, Feng J, Xiao J et al (2021) Determination of uric acid in biological fluids by ceria nanoparticles doped reduced graphene oxide nanocomposite voltammetric sensor. J Electrochem Soc 168:126529
De XJ, Wang CH, Patra J et al (2019) Ionic liquids with various constituent ions to optimize non-enzymatic electrochemical detection properties of graphene electrodes. ACS Sustain Chem Eng 7:16233–16240
Hou J, Xu C, Zhao D, Zhou J (2016) Facile fabrication of hierarchical nanoporous AuAg alloy and its highly sensitive detection towards dopamine and uric acid. Sens Actuators B: Chem 225:241–248
Li Y, Lin H, Peng H et al (2016) A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchim Acta 183:2517–2523
Wang C, Li J, Shi K et al (2016) Graphene coated by polydopamine/multi-walled carbon nanotubes modified electrode for highly selective detection of dopamine and uric acid in the presence of ascorbic acid. J Electroanal Chem 770:56–61
Taleb M, Ivanov R, Bereznev S et al (2017) Ultra-sensitive voltammetric simultaneous determination of dopamine, uric acid and ascorbic acid based on a graphene-coated alumina electrode. Microchim Acta 184:4603–4610
Wang Q, Wang X, Chai Z, Hu W (2013) Low-temperature plasma synthesis of carbon nanotubes and graphene based materials and their fuel cell applications. Chem Soc Rev 42:8821–8834
Kemp KC, Seema H, Saleh M et al (2013) Environmental applications using graphene composites: water remediation and gas adsorption. Nanoscale 5:3149–3171
Megalamani MB, Patil YN, Nandibewoor ST (2023) Electrochemical sensing of carcinogenic p-dimethylamino antipyrine using sensor comprised of eco-friendly MoS2 nanosheets encapsulated by PVA capped Mn doped ZnS nanoparticle. Inorg Chem Commun 151:110617
Patil YN, Megalamani MB, Nandibewoor ST (2023) PVA capped Mn-doped ZnS encapsulated nontoxic MoS2 nano-sheet probe for the sensitive estimation of cardiovascular β-blocking agent acebutolol in biomedical and environmental samples. J Electrochem Soc 170:037505
Korgaonkar K, Pollet BG, Seetharamappa J, Kalanur SS (2023) Ecofriendly synthesis of tenorite (CuO) nanoparticles composite with β-cyclodextrin as an electrochemical sensor for the determination of the anticancer drug phloretin. J Electrochem Soc 170:067505
Dideikin AT, Vul’ AY (2019) Graphene oxide and derivatives: the place in graphene family. Front Phys 6:427630
Karaman C, Karaman O, Atar N, Yola ML (2021) Tailoring of cobalt phosphide anchored nitrogen and sulfur co-doped three dimensional graphene hybrid: boosted electrocatalytic performance towards hydrogen evolution reaction. Electrochim Acta 380:138262
Van Hoa N, Dat PA, Van Hieu N et al (2020) Rapid and efficient synthesis of high-porous reduced graphene oxide/NiCo2S4 nanocomposites for supercapacitor application. Diam Relat Mater 106:107850
Yang Y, Ma G, Huang J et al (2020) Hollow MnO2 spheres/porous reduced graphene oxide as a cathode host for high-performance lithium-sulfur batteries. J Solid State Chem 286:121297
Saisahas K, Soleh A, Somsiri S et al (2022) Electrochemical sensor for methamphetamine detection using laser-induced porous graphene electrode. Nanomaterials 12:73
Green A, Isseroff R, Lin S et al (2017) Synthesis and characterization of iron nanoparticles on partially reduced graphene oxide as a cost-effective catalyst for polymer electrolyte membrane fuel cells. MRS Commun 7:166–172
Karaman C, Aktaş Z, Bayram E et al (2020) Correlation between the molecular structure of reducing agent and pH of graphene oxide dispersion on the formation of 3D-graphene networks. ECS J Solid State Sci Technol 9:071003
Jiang Y, Lu M, Ling X et al (2015) One-step hydrothermal synthesis of three-dimensional porous graphene aerogels/sulfur nanocrystals for lithium–sulfur batteries. J Alloy Compd 645:509–516
Yuan C, Li J, Hou L et al (2013) Polymer-assisted synthesis of a 3D hierarchical porous network-like spinel NiCo2O4 framework towards high-performance electrochemical capacitors. J Mater Chem A 1:11145–11151
Li Y, Zhang L, Hu Z, Yu JC (2015) Synthesis of 3D structured graphene as a high performance catalyst support for methanol electro-oxidation. Nanoscale 7:10896–10902
Gu Y, Wu H, Xiong Z et al (2013) The electrocapacitive properties of hierarchical porous reduced graphene oxide templated by hydrophobic CaCO3 spheres. J Mater Chem A 2:451–459
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Feng H, Cheng R, Zhao X et al (2013) A low-temperature method to produce highly reduced graphene oxide. Nat Commun 4(1):1–8
Zhang WL, Liu YD, Choi HJ (2011) Graphene oxide coated core–shell structured polystyrene microspheres and their electrorheological characteristics under applied electric field. J Mater Chem 21:6916–6921
Worsley MA, Kucheyev SO, Mason HE et al (2012) Mechanically robust 3D graphene macroassembly with high surface area. Chem Commun 48:8428–8430
Wei Y, Liu Y, Xu Z et al (2020) Simultaneous detection of ascorbic acid, dopamine, and uric acid using a novel electrochemical sensor based on palladium nanoparticles/reduced graphene oxide nanocomposite. Int J Anal Chem 2020
Venkatesh K, Muthukutty B, Chen SM et al (2021) Nanomolar level detection of non-steroidal antiandrogen drug flutamide based on ZnMn2O4 nanoparticles decorated porous reduced graphene oxide nanocomposite electrode. J Hazard Mater 405
Mahmood F, Sun Y, Wan C (2021) Biomass-derived porous graphene for electrochemical sensing of dopamine. RSC Adv 11:15410–15415
Mehmandoust M, Çakar S, Özacar M et al (2022) Electrochemical sensor for facile and highly selective determination of antineoplastic agent in real samples using glassy carbon electrode modified by 2D-MoS2 NFs/TiO2 NPs. Top Catal 65:564–576
Zhang S, Fu Y, Sheng Q, Zheng J (2017) Nickel–cobalt double hydroxide nanosheets wrapped amorphous Ni(OH)2 nanoboxes: development of dopamine sensor with enhanced electrochemical properties. New J Chem 41:13076–13084
Veera Manohara Reddy Y, Sravani B, Maseed H et al (2018) Ultrafine Pt–Ni bimetallic nanoparticles anchored on reduced graphene oxide nanocomposites for boosting electrochemical detection of dopamine in biological samples. New J Chem 42:16891–16901
Feng J, Li Q, Cai J et al (2019) Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens Actuators B: Chem 298:126872
Acknowledgements
Thanks are due to SAIF, Karnatak University, Dharwad, for instrumental facilities. One of the authors, Mr. Naveenkumar P Agadi, thanks the Karnatak University for awarding the University Research Fellowship.
Funding
This study received financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, ref. No. 01 (3068)/21/EMR-II dated 23–8-2021.
Author information
Authors and Affiliations
Contributions
Naveenkumar P. Agadi: conceptualization, data curation, software, formal analysis and writing—original draft preparation. Ranjita Tandel: visualization and editing. J. Seetharamappa: supervision, funding acquisition, methodology, investigation, project administration, resources and validation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable (ethical approval is not needed for particular studies as samples were obtained by non-invasive method).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agadi, N.P., Tandel, R. & Seetharamappa, J. Development of a porous reduced graphene oxide modified electrode for simultaneous determination of dopamine and uric acid. Ionics 29, 4863–4873 (2023). https://doi.org/10.1007/s11581-023-05192-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05192-8