Abstract
Due to the urgency of developing high energy density cathode materials in power batteries, as well as the high price and environmental unfriendliness of cobalt, researchers are working on ultra-high nickel low cobalt and ultra-high nickel cobalt-free layered cathode materials. However, the poor cyclic stability and rate performance of this material resulted from structural instability and severe surface/interface side reactions during cycling seriously hinder its practical application. In this paper, LiNi0.9Mn0.1O2 (NM90) cathode material is modified by La2O3 coating, and La3+ doping caused by thermal diffusion is achieved simultaneously. The synergistic enhancement of the two modifications can effectively improve the layered structure and reduce surface/interface side reactions. The dual-modified material shows good structural stability, the La2O3 coating does not fall off and the active material does not appear fragmentation and collapse after 100 cycles. At the same time, the dual-modified material also shows excellent electrochemical performance, with a capacity retention of 83.19% after 100 cycles at 1 C and a reversible capacity of 129.9 mAh g−1 at 10 C. The electrochemical mechanism reveals that the dual-modified material effectively reduces the charge transfer resistance and greatly increases the Li+ diffusion coefficient. It will provide useful reference and technical support for the research of other electrode materials.
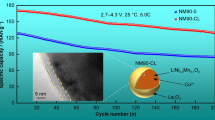
Graphical abstract
The NM90 and La2O3-coated NM90 particles have similar spherical morphology and are formed by the agglomeration of many primary particles; the surface of NM90 material is evenly coated by La2O3 and No obvious boundary between La2O3 and NM90 materials is found; LiNi0.9Mn0.1O2 cathode material coated by 1wt% La2O3 has the best cycle stability.











Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Zhang Y, Li Y, Xia X, Wang X, Gu C, Tu J (2015) High-energy cathode materials for Li-ion batteries: a review of recent developments. Sci China: Technol Sci 58:1809–1828
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4:3243–3262
Mathew V, Kim S, Kang J, Gim J, Song J, Baboo JP, Park W, Ahn D, Han J, Gu L, Wang Y, Hu Y-S, Sun Y-K, Kim J (2015) Amorphous iron phosphate: potential host for various charge carrier ions (vol 6, e138, 2014). NPG Asia Mater 7:e149
Shi C, Hamann T, Takeuchi S, Alexander GV, Nolan AM, Limpert M, Fu Z, O’Neill J, Godbey G, Dura JA, Wachsman ED (2023) 3D asymmetric bilayer garnet-hybridized high-energy-density lithium-sulfur batteries. ACS Appl Mater Interfaces 15:751–760
Jung CH, Kim DH, Eum D, Kim KH, Choi J, Lee J, Kim HH, Kang K, Hong SH (2021) New insight into microstructure engineering of Ni-rich layered oxide cathode for high performance lithium ion batteries. Adv Funct Mater 31:2010095
Tao Q, Wang L, Shi C, Li J, Chen G, Xue Z, Wang J, Wang S, Jin H (2021) Understanding the Ni-rich layered structure materials for high-energy density lithium-ion batteries. Mater Chem Front 5:2607–2622
Hou P, Yin J, Ding M, Huang J, Xu X (2017) Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: advances and perspectives. Small 13:1701802
Shi C, Wang T, Liao X, Qie B, Yang P, Chen M, Wang X, Srinivasan A, Cheng Q, Ye Q, Li AC, Chen X, Yang Y (2019) Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater 17:136–142
Zhang W, Liang L, Zhao F, Liu Y, Hou L, Yuan C (2020) Ni-rich LiNi0.8Co0.1Mn0.1O2 coated with Li-ion conductive Li3PO4 as competitive cathodes for high-energy-density lithium ion batteries. Electrochim Acta 340:135871
Chu M, Huang Z, Zhang T, Wang R, Shao T, Wang C, Zhu W, He L, Chen J, Zhao W, Xiao Y (2021) Enhancing the electrochemical performance and structural stability of Ni-rich layered cathode materials via dual-site doping. ACS Appl Mater Interfaces 13:19950–19958
Wang J, Liu C, Xu G, Miao C, Wen M, Xu M, Wang C, Xiao W (2022) Strengthened the structural stability of in-situ F-doping Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries. Chem Eng J 438:135537
Qiu L, Xiang W, Tian W, Xu CL, Li YC, Wu ZG, Chen TR, Jia K, Wang D, He FR, Guo XD (2019) Polyanion and cation co-doping stabilized Ni-rich Ni–Co–Al material as cathode with enhanced electrochemical performance for Li-ion battery. Nano Energy 63:103818
Park KJ, Jung HG, Kuo LY, Kaghazchi P, Yoon CS, Sun YK (2018) Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries. Adv Energy Mater 8:201801202
Park GT, Ryu HH, Park NY, Yoon CS, Sun YK (2019) Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage. J Power Sources 442:227242
Zhang JT, Tan XH, Guo LM, Jiang Y, Liu SN, Wang HF, Kang XH, Chu WG (2019) Controllable formation of lithium carbonate surface phase during synthesis of nickel-rich LiNi0.9Mn0.1O2 in air and its protection role in electrochemical reaction. J Alloys Compd 771:42–50
Li N, Sallis S, Papp JK, Wei J, McCloskey B, Yang W, Tong W (2019) Unraveling the cationic and anionic redox reactions in a conventional layered oxide cathode. ACS Energy Lett 4:2836–2842
Tian J, Su Y, Wu F, Xu S, Chen F, Chen R, Li Q, Li J, Sun F, Chen S (2016) High-rate and cycling-stable nickel-rich cathode materials with enhanced li+ diffusion pathway. ACS Appl Mater Interfaces 8:582–587
Xia Y, Zheng J, Wang C, Gu M (2018) Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 49:434–452
Yin S, Deng W, Chen J, Gao X, Zou G, Hou H, Ji X (2021) Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 83:105854
Shobana MK (2019) Metal oxide coated cathode materials for Li ion batteries – a review. J Alloys Compd 802:477–487
Zhang Z, Zhou P, Meng H, Chen C, Cheng F, Tao Z, Chen J (2017) Amorphous Zr(OH)4 coated LiNi0.915Co0.075Al0.01O2 cathode material with enhanced electrochemical performance for lithium ion batteries. J Energy Chem 26:481–487
Tran TTB, Park EJ, Kim HI, Jang HJ, Son JT (2022) Solvent-free dry cyclized polyacrylonitrile-coated LiNi0.87Co0.13O2 cathode for improving the electrochemical performance of Li-ion batteries. Mater Chem Phys 290:126590
Yu H, Zhu H, Yang Z, Liu M, Jiang H, Li C (2021) Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes. Chem Eng J 412:128625
Xie Y, Guo F, Zhang Y (2022) One-step calcination reaction to synthesize Li2MnO3 coating layers for LiNi0.8Co0.1Mn0.1O2 to improve cycling performances under high-voltage for Li-ion batteries. Appl Surf Sci 595:153479
Zhou H, Zhou F, Shi S, Yang W, Song Z (2020) Influence of working temperature on the electrochemical characteristics of Al2O3-coated LiNi0.8Co0.1Mn0.1O2 cathode materials for Li-ion batteries. J Alloys Compd 847:156412
Liang L, Hu G, Jiang F, Cao Y (2016) Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J Alloys Compd 657:570–581
Zheng J, Gu M, Xiao J, Polzin BJ, Yan P, Chen X, Wang C, Zhang J-G (2014) Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials. Chem Mater 26:6320–6327
Jan SS, Nurgul S, Shi X, Xia H, Pang H (2014) Improvement of electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by graphene nanosheets modification. Electrochim Acta 149:86–93
Han Y, Heng S, Wang Y, Qu Q, Zheng H (2020) Anchoring interfacial nickel cations on single-crystal LiNi0.8Co0.1Mn0.1O2 cathode surface via controllable electron transfer. ACS Energy Lett 5:2421–2433
Kang J-G, Kim Y-I, Won Cho D, Sohn Y (2015) Synthesis and physicochemical properties of La(OH)3 and La2O3 nanostructures. Mater Sci Semicond Process 40:737–743
Zhao S, Zhou H, Zhou T, Zhang Z, Lin P, Ren L (2013) The oxidation resistance and ignition temperature of AZ31 magnesium alloy with additions of La2O3 and La. Corros Sci 67:75–81
Fan H-Q, Xia D-H, Li M-C, Li Q (2017) Self-assembled (3-mercaptopropyl)trimethoxylsilane film modified with La2O3 nanoparticles for brass corrosion protection in NaCl solution. J Alloys Compd 702:60–67
Zhang Y, Dong P, Zhang M, Sun X, Yu X, Song J, Meng Q, Li X, Zhang Y (2017) Combustion combined with ball milling to produce nanoscale La2O3 coated on LiMn2O4 for optimized Li-ion storage performance at high temperature. J Appl Electrochem 48:135–145
Mohan P, Kalaignan GP (2013) Electrochemical performance of La2O3-coated layered LiNiO2 cathode materials for rechargeable lithium-ion batteries. Ionics 19:895–902
Liu J (2018) Improvement of high-voltage electrochemical performance of surface modified LiNi0.6Co0.2Mn0.2O2 cathode by La2O3 coating. Int J Electrochem Sci 13:9816–9825
Li W, Erickson EM, Manthiram A (2020) High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat Energy 5:26–34
Li W, Lee S, Manthiram A (2020) High-nickel NMA: a cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv Mater 32:2002718
Turcheniuk K, Bondarev D, Singhal V, Yushin G (2018) Ten years left to redesign lithium-ion batteries Reserves of rare metals used in electric-vehicle cells are dwindling, so boost research on iron and silicon alternatives, urge Kostiantyn Turcheniuk and colleagues. Nature 559:467–470
Hua W-B, Guo X-D, Zheng Z, Wang Y-J, Zhong B-H, Fang B, Wang J-Z, Chou S-L, Liu H (2015) Uncovering a facile large-scale synthesis of LiNi1/3Co1/3Mn1/3O2 nanoflowers for high power lithium-ion batteries. J Power Sources 275:200–206
Yang Z, Guo X, Xiang W, Hua W, Zhang J, He F, Wang K, Xiao Y, Zhong B (2017) K-doped layered LiNi0.5Co0.2Mn0.3O2 cathode material: towards the superior rate capability and cycling performance. J Alloys Compd 699:358–365
Cheng C, Tan L, Liu H, Huang X (2011) High rate performances of the cathode material LiNi1/3Co1/3Mn1/3O2 synthesized using low temperature hydroxide precipitation. Mater Res Bull 46:2032–2035
Zhao L, Chen G, Weng Y, Yan T, Shi L, An Z, Zhang D (2020) Precise Al2O3 coating on LiNi0.5Co0.2Mn0.3O2 by atomic layer deposition restrains the shuttle effect of transition metals in Li-ion capacitors. Chem Eng J 401:126138
Kang S, Qin H, Fang Y, Li X, Wang Y (2014) Preparation and electrochemical performance of yttrium-doped Li[Li0.20Mn0.534Ni0.133Co0.133]O2 as cathode material for lithium-ion batteries. Electrochim Acta 144:22–30
Wu F, Tian J, Su Y, Guan Y, Jin Y, Wang Z, He T, Bao L, Chen S (2014) Lithium-active molybdenum trioxide coated LiNi0.5Co0.2Mn0.3O2 cathode material with enhanced electrochemical properties for lithium-ion batteries. J Power Sources 269:747–754
Xu CL, Xiang W, Wu ZG, Li YC, Xu YD, Hua WB, Guo XD, Zhang XB, Zhong BH (2018) A comparative study of crystalline and amorphous Li0.5La0.5TiO3 as surface coating layers to enhance the electrochemical performance of LiNi0.815Co0.15Al0.035O2 cathode. J Alloys Compd 740:428–435
Sun G, Yin X, Yang W, Zhang J, Du Q, Ma Z, Shao G, Wang Z-B (2018) Synergistic effects of ion doping and surface-modifying for lithium transition-metal oxide: synthesis and characterization of La2O3-modified LiNi1/3Co1/3Mn1/3O2. Electrochim Acta 272:11–21
Li Y-C, Xiang W, Xiao Y, Wu Z-G, Xu C-L, Xu W, Xu Y-D, Wu C, Yang Z-G, Guo X-D (2019) Synergy of doping and coating induced heterogeneous structure and concentration gradient in Ni-rich cathode for enhanced electrochemical performance. J Power Sources 423:144–151
Li X, Cheng L, Chen L, Huang B, Yang J, Li Y, Li W (2022) Stabilizing the surface of LiNi0.815Co0.15Al0.035O2 by a facile pre-oxidation-coating strategy on the precursor. Solid State Ionics 386:116028
He R, Wei A, Bai X, Zhang L, Li X, Jinping M, Zhang X, Ge J, Liu Z (2021) Enhanced cycling performance of Li ion batteries based on Ni-rich cathode materials with LaPO4/Li3PO4 co-modification. Ceram Int 47:34585–34594
Yao S, Wang Y, Liang Y, Yu H, Majeed A, Shen X, Li T, Qin S (2021) Modified polysulfides conversion catalysis and confinement by employing La2O3 nanorods in high performance lithium-sulfur batteries. Ceram Int 47:27012–27021
Yu H, Cao Y, Chen L, Hu Y, Duan X, Dai S, Li C, Jiang H (2021) Surface enrichment and diffusion enabling gradient-doping and coating of Ni-rich cathode toward Li-ion batteries. Nat Commun 12:4564
Wang R, Zhang T, Zhang Q, Zheng M, Xu K, Yan W (2019) Enhanced electrochemical performance of La and F co-modified Ni-rich cathode. Ionics 26:1165–1171
Lv Y, Huang S, Lu S, Ding W, Yu X, Liang G, Zou J, Kang F, Zhang J, Cao Y (2022) B2O3/LiBO2 dual-modification layer stabilized Ni-rich cathode for lithium-ion battery. J Power Sources 536:231510
Kim SB, Kim H, Park DH, Kim JH, Shin JH, Jang JS, Moon SH, Choi JH, Park KW (2021) Li-ion diffusivity and electrochemical performance of Ni-rich cathode material doped with fluoride ions. J Power Sources 506:230219
Li Y, Huang Y, Zheng Y, Huang R, Yao J (2019) Facile and efficient synthesis of α-Fe2O3 nanocrystals by glucose-assisted thermal decomposition method and its application in lithium ion batteries. J Power Sources 416:62–71
Yao J, Wu J, Yang Y, Xiao S, Li Y (2020) Lithium storage performance of coralline-like FeMnO3 anode materials prepared by a facile chemical co-precipitation method. J Alloys Compd 848:156444
Funding
This work was financially supported the National Science Foundation of China (Grant No. 22169007), the Science and Technology Major Project of Guangxi (Grant No. AA19046001), the Open Research Fund of Guangxi Key Laboratory of Electrochemical and Magnetochemical Functional Materials (Grant No. EMFM20201105, EMFM20181119), and the Characteristic Innovation Projects of Universities in Guangdong Province (Grant No. 2022KTSCX324).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• LiNi0.9Mn0.1O2 and La2O3-coated LiNi0.9Mn0.1O2 cathode materials are successfully prepared by high temperature solid phase sintering.
• La2O3-coated LiNi0.9Mn0.1O2 cathode material has excellent magnification performance and cycle stability.
• It provides an effective surface modification method to obtain Ni-rich cobalt-free layered cathode materials with high rate and cycle performance.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, J., Wei, Y., Hu, B. et al. Ultra-high nickel cobalt-free layered cathode material NM90 for power batteries modified collaboratively by La2O3 coating and La3+ doping. Ionics 29, 2549–2561 (2023). https://doi.org/10.1007/s11581-023-05010-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05010-1