Abstract
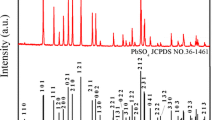
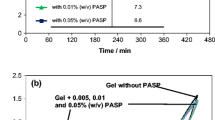
This article investigates the influence of sodium polyaspartate (PASP), used as an additive in the negative active material (NAM) on the performance of lead-acid batteries, including battery capacity at different discharge/charge rates, fast charge performance, high rate discharge performance, and cycling performance. The addition of PASP to NAM can prevent lead sulfate accumulation and refine the size of lead sulfate crystals making them easier to restore during the charging process, thus enhancing the utilization of NAM. Consequently, the charge and discharge capacity and the cycling performance are also improved. Many remarkable improvements of the performance of lead-acid batteries have been observed during the battery tests. The obtained results show that addition of 0.4–0.9 % PASP additive to NAM yields highest discharge capacity compared to that of regular batteries, exceeding by about 21–29 % of the 3-h rate capacity on average. Other performance parameters also show a high superiority trend as compared to batteries without additive and the additive loading level ranges between 0.4 and 4 %.








Similar content being viewed by others
References
Ban I, Yanaguchi Y, Nakayama Y, Hirai N, Hara S (2002) J Power Sources 107:167–172
Francia C, Maja M, Spineli P, Saez F, Martinez B, Marin D (2000) J Power Sources 85:102–109
Hirai N, Tabayashi D, Shiota M, Tanaka T (2004) J Power Sources 133:32–38
Pavlov D, Myrvold BO, Rogachev T, Martrakava M (2000) J Power Sources 85:79–91
Boden DP (2004) Hammond lead products, a division of hammond group, 2323 165th Street, Hammond, IN 46325. USA J Power Sources 133:47–51
Logeshkumar S, Manoharan R (2014) Electrochim Acta 144:147–153
Saez F, Martinez B (2001) J Power Sources 95:174–190
Shiomi M (1997) J Power Sources 64:147–152
Calabek M, Micka K, Krivak P et al (2006) J Power Sources 158:864–867
Chen XJ, Jiang LX, Peng B, Zhang ZA, Lai YQ, Li J, Liu YX (2012) J Central South Univ 6:2023–2028
Pavlov D (1997) J Power Sources 64:131–137
Bullock KR (2010) J Power Sources 195:4513–4519
Hong B, Jiang L, Xue H, Liu F, Jia M, Li J, Liu Y (2014) J Power Sources 270:332–341
Burke E, Guo Y, Colon L, Rahima M, Veis A, Nancollas GH (2000) Colloids Surf B: Biointerfaces 17:49–57
Ross R, Low K, Shannon J (1997) Mater Perform 36(4):53
Schmitt G, Saleh AO (2000) Mater Perform 39(8):62
Hater W, Mayer B, Schweinsberg M (2000) Power Plant Chem 2:12
Freeman M, Hann W, Paik Y, Swift G, Patent No. 5,531,934, 7 February 1996
Tang J, Fu S, Emmons D, Patent No. 6,022,401, 2 August 2000
Petkova G, Nikolov P, Pavlov D (2006) J Power Sources 158:841–845
Acknowledgments
This work was funded by the Natural Science Foundation Project of Guangdong Province (No. S2013010014847), Production, Education & Research Combining Project of Guangdong Province and Ministry of Education (No. 2011B090400560), Science and Technology Project of Guangzhou (No. 11A92091438), Science and Technology Project of Panyu District (No. 2010-zhuan-12-2), Smelter Group in Zhuzhou City, Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, X., Cai, J. et al. Influence of sodium polyaspartate added to the negative active material on the electrochemical behavior of lead-acid batteries. Ionics 21, 2693–2700 (2015). https://doi.org/10.1007/s11581-015-1446-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1446-7