Abstract
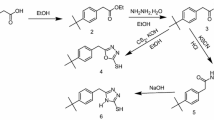
Methyl 3-((2-mercaptophenyl) imino) butanoate (MMPB) was designed and synthesized as a corrosion inhibitor, which is functionalized with adjacent azole and thiol groups and a carboxylate tail. The inhibition efficiency of this compound has been investigated in different concentrations of HCl solutions. Then, the effect of temperature and inhibitor concentration was studied for further discussion about inhibition mechanism. In addition to potentiodynamic and electrochemical impedance spectroscopy, galvanic measurements were also realized for better explanation of interaction between inhibitor and metal surface. For this purpose, identical steel electrodes were immersed in separate test solutions with and without inhibitor, and then coupled to each other. The assessment of corrosion rate was realized with quantitative analysis of iron content in immersion test solutions. The corrosion current densities (i corr) were 20.40 and 200.30 μA cm−2, in the presence of 10 mM inhibitor and inhibitor-free test solutions, respectively. The energy barrier values against corrosion were also calculated in the presence and absence of inhibitor, with the help of surface coverage ratio and i corr values for different temperatures.














Similar content being viewed by others
References
Torres VV, Rayol VA, Magalhães M et al (2014) Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corros Sci 79:108–118
Obot IB, Ebenso EE, Kabanda MM (2013) Metronidazole as environmentally safe corrosion inhibitor for mild steel in 0.5 M HCl: Experimental and theoretical investigation. J Environ Chem Eng 1:431–439
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62:113–116
Negm NA, Kandile NG, Aiad IA et al (2011) New eco-friendly cationic surfactants: synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surf A 391:224–233
Moretti G, Guidi F, Grion G (2004) Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros Sci 46:387–403
Ghareba S, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of ‘green’ corrosion inhibitors. Corros Sci 52:2104–2113
Deng Q, Ding NN, Wei XL et al (2012) Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros Sci 64:64–73
Fragoza-Mar L, Olivares-Xometl O, Domínguez-Aguilar MA et al (2012) Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution. Corros Sci 61:171–184
Flores EA, Olivares O, Likhanova NV et al (2011) Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution. Corros Sci 53:3899–3913
Zhang F, Tang Y, Cao Z et al (2012) Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid. Corros Sci 61:1–9
Tao Z, Zhang S, Li W et al (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51:2588–2595
Karthikaiselvi R, Subhashini S (2012) The water soluble composite poly (vinylpyrrolidone–methylaniline): A new class of corrosion inhibitors of mild steel in hydrochloric acid media. Arab. J. Chem. In Press
Yadav M, Behera D, Sharma U (2012) Nontoxic corrosion inhibitors for N80 steel in hydrochloric acid. Arab. J. Chem. In Press
Zhang S, Tao Z, Liao S et al (2010) Substitutional adsorption isotherms and corrosion inhibitive properties of some oxadiazol-triazole derivative in acidic solution. Corros Sci 52:3126–3132
Ita BI, Offiong OE (2001) The study of the inhibitory properties of benzoin, benzil, benzoin-(4-phenylthiosemicarbazone) and benzil-(4-phenylthiosemicarbazone) on the corrosion of mild steel in hydrochloric acid. Mater Chem Phys 70:330–335
Singh AK, Shukla SK, Singh M et al (2011) Inhibitive effect of ceftazidime on corrosion of mild steel in hydrochloric acid solution. Mater Chem Phys 129:68–76
Deng Q, Shi HW, Ding NN et al (2012) Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros Sci 57:220–227
Migahed MA, Abdul-Raheim AM, Atta AM et al (2010) Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly (ethylene terephthalate). Mater Chem Phys 121:208–214
Deng Q, He XP, Shi HW et al (2012) Concise Cu1-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction ligation remarkably enhances the corrosion inhibitive potency of natural amino acids for mild steel in HCl. Ind Eng Chem Res 51:7160–7169
Cai L, Fu Q, Shi R et al (2014) Pungent copper surface resists acid corrosion in strong HCl solutions. Ind Eng Chem Res 53:64–69
Zaferani SH, Sharifi M, Zaarei D et al (2013) Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes—a review. J Environ Chem Eng 1:652–657
Mahgoub FM, Abdel-Nabey BA, El-Samadisy YA (2010) Adopting a multipurpose inhibitor to control corrosion of ferrous alloys in cooling water systems. Mater Chem Phys 120:104–108
Liu F, Lu X, Yang W et al (2013) Optimizations of inhibitors compounding and applied conditions in simulated circulating cooling water system. Desalination 313:18–27
Azghandi MV, Davoodi A, Farzi GA et al (2012) Water-base acrylic terpolymer as a corrosion inhibitor for SAE1018 in simulated sour petroleum solution in stagnant and hydrodynamic conditions. Corros Sci 64:44–54
Abd-El-Khalek DE, Abd-El-Nabey BA (2013) Evaluation of sodium hexametaphosphate as scale and corrosion inhibitor in cooling water using electrochemical techniques. Desalination 311:227–233
Dkhireche N, Dahami A, Rochdi A et al (2013) Corrosion and scale inhibition of low carbon steel in cooling water system by 2-propargyl-5-o-hydroxyphenyltetrazole. J Ind Eng Chem 19:1996–2003
Wilhelm EJ, Ereneta VG (1973) Corrosion inhibitor control in aqueous solutions evaluated with galvanic couples. Corros Sci 13:1003–1017
Izquierdo J, Nagy L, Santana JJ et al (2011) A novel microelectrochemical strategy for the study of corrosion inhibitors employing the scanning vibrating electrode technique and dual potentiometric/amperometric operation in scanning electrochemical microscopy: application to the study of the cathodic inhibition by benzotriazole of the galvanic corrosion of copper coupled to iron. Electrochim Acta 58:707–716
Ai JZ, Guo XP, Chen ZY (2006) The adsorption behavior and corrosion inhibition mechanism of anionic inhibitor on galvanic electrode in 1 % NaCl solution. Appl Surf Sci 253:683–688
Kallip S, Bastos AC, Yasakau KA et al (2012) Synergistic corrosion inhibition on galvanically coupled metallic materials. Electrochem Commun 20:101–104
Moretti G, Guidi F, Fabris F (2013) Corrosion inhibition of the mild steel in 0.5 M HCl by 2-butyl-hexahydropyrrolo [1,2-b] [1,2] oxazole. Corros Sci 76:206–218
Fouda AS, Ellithy AS (2009) Inhibition effect of 4-phenylthiazole derivatives on corrosion of 304L stainless steel in HCl solution. Corros Sci 51:868–875
Musa AY, Kadhum AAH, Mohamad AB et al (2010) On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1,2,4-trizole-3-thiol. Corros Sci 52:526–533
Wang HL, Liu RB, Xin J (2004) Inhibiting effects of some mercapto-triazole derivatives on the corrosion of mild steel in 1.0 M HCl medium. Corros Sci 46:2455–2466
Cheng XL, Ma HY, Chen SH et al (1998) Corrosion of stainless steels in acid solutions with organic sulfur-containing compounds. Corros Sci 41:321–333
Popova A, Sokolova E, Raicheva S et al (2003) AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci 45:33–58
Wang HL, Fan HB, Zheng JS (2003) Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater Chem Phys 77:655–661
Mahdavian M, Ashhari S (2010) Corrosion inhibition performance of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole compounds for protection of mild steel in hydrochloric acid solution. Electrochim Acta 55:1720–1724
Álvarez-Bustamante R, Negrón-Silva G, Abreu-Quijano M et al (2009) Electrochemical study of 2-mercaptoimidazole as a novel corrosion inhibitor for steels. Electrochim Acta 54:5393–5399
Sherif EM (2014) Corrosion inhibition in 2.0 M sulfuric acid solutions of high strength maraging steel by aminophenyl tetrazole as a corrosion inhibitor. Appl Surf Sci 292:190–196
Bentiss F, Lebrini M, Vezin H et al (2004) Experimental and theoretical study of 3-pyridyl-substituted 1,2,4-thiadiazole and 1,3,4-thiadiazole as corrosion inhibitors of mild steel in acidic media. Mater Chem Phys 87:18–23
Bentiss F, Lebrini M, Lagrenée M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis (n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Elkadi L, Mernari B, Traisnel M et al (2000) The inhibition action of 3,6-bis (2-methoxyphenyl)-1,2-dihydro-1,2,4,5-tetrazine on the corrosion of mild steel in acidic media. Corros Sci 42:703–719
Bentiss F, Traisnel M, Lagrenee M (2000) The substituted 1,3,4-oxadiazoles: a new class of corrosion inhibitors of mild steel in acidic media. Corros Sci 42:127–146
El Azhar M, Mernari B, Traisnel M et al (2001) Corrosion inhibition of mild steel by the new class of inhibitors [2,5-bis (n-pyridyl)-1,3,4-thiadiazoles] in acidic media. Corros Sci 43:2229–2238
Bentiss F, Traisnel M, Vezin H et al (2004) 2,5-Bis (4-dimethylaminophenyl)-1,3,4-oxadiazole and 2,5-bis (4-dimethylaminophenyl)-1,3,4-thiadiazole as corrosion inhibitors for mild steel in acidic media. Corros Sci 46:2781–2792
Al-Sarawy AA, Fouda AS, Shehab El-Dein WA (2008) Some thiazole derivatives as corrosion inhibitors for carbon steel in acidic medium. Desalination 229:279–293
Ali SA, Saeed MT, Rahman SU (2003) The isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic medium. Corros Sci 45:253–266
Tao Z, Zhang S, Li W et al (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51:2588–2595
Ali SA, Al-Muallem HA, Saeed MT et al (2008) Hydrophobic-tailed bicycloisoxazolidines: a comparative study of the newly synthesized compounds on the inhibition of mild steel corrosion in hydrochloric and sulfuric acid media. Corros Sci 50:664–675
Li W, He Q, Pei C et al (2007) Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta 52:6386–6394
Manivel A, Ramkumar S, Wu JJ et al (2014) Exploration of (S)-4,5,6,7-tetrahydrobenzo [d] thiazole-2,6-diamine as feasible corrosion inhibitor for mild steel in acidic media. J Environ Chem Eng 2:463–470
Hassan HH, Abdelghani E, Amin MA (2007) Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives: part I. Polarization and EIS studies. Electrochim Acta 52:6359–6366
Markhali BP, Naderi R, Mahdavian M et al (2013) Electrochemical impedance spectroscopy and electrochemical noise measurements as tools to evaluate corrosion inhibition of azole compounds on stainless steel in acidic media. Corros Sci 75:269–279
Ali SA, Al-Muallem HA, Rahman SU et al (2008) Bis-isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic media. Corros Sci 50:3070–3077
Abboud Y, Abourriche A, Saffaj T et al (2006) The inhibition of mild steel corrosion in acidic medium by 2,2′-bis (benzimidazole). Appl Surf Sci 252:8178–8184
Dhayabaran VV, Lydia IS, Merlin JP et al (2004) Inhibition of corrosion of commercial mild steel in presence of tetrazole derivatives in acid medium. Ionics 10:123–125
Tansug G, Tuken T, Kicir N et al (2014) Investigation of 2-aminoethanethiol as corrosion inhibitor for steel using response surface methodology (RSM). Ionics 20:287–294
Kesavan D, Tamizh MM, Gopiraman M et al (2012) Physicochemical studies of 4-substituted N-(2-mercaptophenyl)-salicylideneimines: corrosion inhibition of mild steel in an acid medium. J Surfact Deterg 15:567–576
Al-Amiery AA, Kadhum AAH, Mohamad AB et al (2013) A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials 6:1420–1431
Popova A, Christov M, Zwetanova A (2007) Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros Sci 49:2131–2143
Popova A, Christov M (2006) Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros Sci 48:3208–3221
Tang Y, Zhang F, Hu S et al (2013) Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: gravimetric, electrochemical, SEM and XPS studies. Corros Sci 74:271–282
Ashassi-Sorkhabi H, Seifzadeh D, Hosseini MG (2008) EN, EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1 M HCl solution. Corros Sci 50:3363–3370
Saliyan VR, Adhikari AV (2008) Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl) quinolin-4-yl] thio} propanohydrazide as an effective inhibitor of mild steel corrosion in HCl solution. Corros Sci 50:55–61
Tüken T, Demir F, Kıcır N et al (2012) Inhibition effect of 1-ethyl-3-methylimidazolium dicyanamide against steel corrosion. Corros Sci 59:110–118
El-Rehim SSA, Refaey SAM, Taha F et al (2001) Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenol and 2-cyanomethyl benzothiazole. J Appl Electrochem 31:429–435
Kabel KI, Zakaria K, Abbas MA, Khamis EA (2014) Assessment of corrosion inhibitive behavior of 2-aminothiophenol derivatives on carbon steel in 1 M HCl. J. Ind. Eng. Chem. in press
Kosari A, Momeni M, Parvizi R et al (2011) Theoretical and electrochemical assessment of inhibitive behavior of some thiophenol derivatives on mild steel in HCl. Corros Sci 53:3058–3067
Wang IS (2007) Corrosion research trends. Nova Science, New York
Tansuğ G, Tüken T, Giray ES et al (2014) A new corrosion inhibitor for copper protection. Corros Sci 84:21–29
Bockris J, Reddy AKN, Gamboa-Aldeco M (2000) Modern electrochemistry, vol 2A. Kluwer Academic/Plenum, New York
Hsu CH, Mansfeld F (2001) Technical note: concerning the conversation of the constant phase element parameter Y0 into a capacitance. Corrosion 57:747–748
Lemlein R (1973) Diplomarbeit, Institut fur Physikalische Chemie und Electrochemie III der Universitat (TH) Karlsruhe, Germany
Lorenz WJ (1965) Der einfluss von halogenidionen auf die anodische auflosung des eisens. Corros Sci 5:121–131
Darwish NA, Hilbert F, Lorenz WJ et al (1973) The influence of chloride ions on the kinetics of iron dissolution. Electrochim Acta 18:421–425
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tansuğ, G., Tüken, T., Sığırcık, G. et al. Methyl 3-((2-mercaptophenyl) imino) butanoate as an effective inhibitor against steel corrosion in HCl solution. Ionics 21, 1461–1475 (2015). https://doi.org/10.1007/s11581-014-1296-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1296-8