Abstract
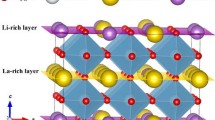
To date, the fastest lithium ion-conducting solid electrolytes known are the perovskite-type ABO3 oxide, with A = Li, La and B = Ti, lithium lanthanum titanate (LLTO) \({\rm Li}_{3x} {\rm La}_{\left( {{2 \mathord{\left/ {\vphantom {2 3}} \right. \kern-\nulldelimiterspace} 3}} \right) - x} \Box_{\left( {{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-\nulldelimiterspace} 3}} \right) - x} {\rm TiO}_3 \) and its structurally related materials. In this formula, \(\Box\) represents the vacancy. These materials have attracted much attention due to their application in lithium ion batteries used as energy sources in microelectronic and information technologies. In addition to the well-established simple cubic, tetragonal and orthorhombic perovskite type distorted cell structures, the hexagonal unit cell was reported in a recent study for Li0.5 La0.5 TiO3 − δ , \(\left( {0 \le \delta \le 0.06} \right)\). We investigated the ionic conductivity in hexagonal \({\rm La}_{0.5} {\rm Li}_{0.5}\- {\rm TiO}_3 \) by molecular dynamics. We confirmed that ionic conductivity in this compound is due to the motion of lithium ions. We show that both Arrhenius and Vogel–Tamman–Fulcher-type relationships could be used to express the high-temperature conductivity of this compound. From our results, hexagonal LLTO exhibits almost 1.7–1.9 ×10 − 3 S cm − 1 at room temperature. Thus, due to its high ionic conductivity, this compound is expected to show some advantages in comparison with the best conductors of this family, for usual applications of ionic conductors.







Similar content being viewed by others
Notes
The charge density cutoff energy is usually six to 12 times greater than the wave function cutoff energy. In this work, this ratio is 8.
References
Belous AG, Gavrilova LG, Polianetskaya SV, Makarova ZY, Chalyi VP (1984) Stabilisation of perovskite structure in lanthanum titanate(in Russian). Ukr Khim Zh 50(5):460–461
Belous AG, Butko VI, Novitskaya GN, Polianetskaya SV, Homenko BS, Poplavko YM (1986) (in Russian). Ukr Fiz Z 31(4):576–581
Inaguma Y, Liquan C, Itoh M, Nakamura T, Uchida T, Ikuta H, Wakihara M (1993) High ionic conductivity in lithium lanthanum titanate. Solid State Commun 86(10):689–693
Fourquet JL, Duroy H, Crosnier-Lopez MP (1996) Structural and microstructural studies of the series \(La_{{2 \mathord{\left/ {\vphantom {2 3}} \right. \kern-\nulldelimiterspace} 3} - x} Li_{3x} \Box _{{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-\nulldelimiterspace} 3} - 2x} TiO_3 \). J Solid State Chem 127(0385):283–294
Stramare S, Thangadurai V, Weppner W (2003) Lithium lanthanum titanates: a review. Chem Mater 15(21):3974–3990
Alonso JA, Sanz J, Santamaría J, León C, Várez A, Fernández-Díaz MT (2000) On the location of Li + cations in the fast Li-cation conductor La 0.5 Li 0.5 TiO 3 perovskite. Angew Chem Int Ed 39(3):619–621
Varez A, Fernández-Díaz MT, Sanz J (2004) Rhombohedral-cubic transition in Li 0.2 Na 0.3 La 0.5 TiO 3 perovskite. J Solid State Chem 177:4665–4671
Varez A, Fernández-Díaz MT, Alonso JA, Sanz J (2005) Structure of fast ion conductors \(Li_{3x} La_{{2 \mathord{\left/ {\vphantom {2 3}} \right. \kern-\nulldelimiterspace} 3} - x} TiO_3 \) deduced from powder neutron diffraction experiments. Chem Mater 17(9):2404–2412
Sanjuán ML, Laguna MA, Belous AG, V’yunov OI (2005) On the local structure and lithium dynamics of \(La_{0.5} \left( {Li,Na} \right)_{0.5} TiO_3 \) ionic conductors. A Raman study. Chem Mater 17(23):5862–5866
Aroyo MI et al (2006) Bilbao crystallographic server: I. databases and crystallographic computing program. Z Kristallogr 221(1):15–27. http://www.cryst.ehu.es/cryst/
Kokalj A (2003) Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput Mater Sci 28:155. http://www.xcrysden.org/
Giannozzi P, Baroni S et al (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502. V. 4. 0. 5. http://www.pwscf.org/
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41:7892
Laasonen K, Car R, Lee C, Vanderbilt D (1991) Implementation of ultra-soft pseudopotentials in ab-initio molecular dynamics. Phys Rev B 43:6796
Laasonen K, Pasquarello A, Lee C, Car R, Vanderbilt D (1993) Car–Parrinello molecular dynamics with Vanderbilt’s ultrasoft pseudopotentials. Phys Rev B 47:10142
Perdew JP (1991) In: Electronic structure of solids ’91. Ziesche P, Eschrig H (eds) vol 11. Akademie, Berlin
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Sitamtze Youmbi B, Zekeng S, Domngang S (2010) Zero point corrections and entropy effect on ab-initio equation-of-state: a case study on bulk titanium. J Phys Chem Solids 71(9):1206–1212
Morkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(1):5188–5192
Marx D, Hutter J (2000) Ab initio molecular dynamics: theory and implementation, modern methods and algorithms of quantum chemistry. In: Grotendorst J (ed) Proceedings second edition, John Von Neumann Institute for Computing, Julich, NIC series, vol 3. John Von Neumann Institute for Computing, Jülich, pp 329–477
Fortal’nova EA, Mosunov AV, Safronenko MG, Venskovskii NU, Politova ED (2006) Electrical properties of \(\left( {La_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} Li_{{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-\nulldelimiterspace} 3} + x} } \right)TiO_3 \). Inorg Mater 42(4):393–398
Maruyama Y, Ogawa H, Kamimura M, Ono S, Kobayashi M (2008) Dynamical properties and electronic structure of (LaLi)TiO 3 conductors. IONICS 14(5):357–361
Bohnke O, Bohnke C, Fourquet JL (1996) Mechanism of ionic conduction and electrochemical intercalation of lithium into the perovskite lanthanum lithium titanate. Solid State Ion 91:21–31
Rivera A, León C, Santamará J, Várez A, V’yunov O, Belous AG, Alonso JA, Sanz J (2002) Percolation-limited ionic diffusion in Li 0.5 − x Na x La 0.5 TiO 3 perovskites (0 ≤ x ≤ 0.5). Chem Mater 14(12):5148–5152
Léon C, Lucía ML, Santamaría J, París MA, Sanz J, Várez A (1996) Electrical conductivity relaxation and nuclear magnetic resonance of Li conducting La 0.5 Li 0.5 TiO 3 . Phys Rev B 54(1):184–189
Inaguma Y, Yu J, Katsumata T, Itoh M (1997) Lithium ion conductivity in a perovskite lanthanum lithium crystal. J Ceram Soc Jpn 105:548–550
Harada Y, Ishigaki T, Kawai H, Kuwano J (1998) Lithium ion conductivity of polycrystalline perovskite La 0.67 − x Li 3x TiO 3 with ordered and disordered arrangements of the A-site ions. Solid State Ion 108(1–4):407–413
Inaguma Y, Chen L, Itoh M, Nakamura T (1994) Candidate compounds with perovskite structure for high lithium ionic conductivity. Solid State Ion 70/71(1):196–202
Inaguma Y, Itoh M (1996) Influences of carrier concentration and site percolation on lithium ion conductivity in perovskite-type oxides. Solid State Ion 86–88:257–260
Acknowledgements
The authors highly appreciate the computer assistance from the Euro-Graduation-Access(EG@) Consortium. We also gratefully thank Dr. Emire MAGA for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youmbi, B.S., Zékeng, S., Domngang, S. et al. An ab initio molecular dynamics study of ionic conductivity in hexagonal lithium lanthanum titanate oxide La 0.5 Li 0.5 TiO 3 . Ionics 18, 371–377 (2012). https://doi.org/10.1007/s11581-012-0662-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0662-7