Abstract
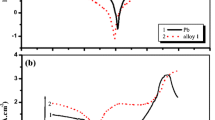
In this work, the application of ionic liquids (ILs)—mono-, bicycyclohexyl, monohexyl and tetrahexyl ammonium hydrogen sulphate—as electrolyte additives on the electrochemical performance of lead acid batteries is proposed. The electrochemical behaviour of Pb–1.66% Sb–0.24% Sn alloy in sulphuric acid solution is investigated in the presence of the mentioned ILs with different numbers of alkyl or cycloalkyl chains. Particularly, the hydrogen and oxygen evolution potential and anodic layer characteristics were studied using cyclic and linear sweep voltammetric methods. The obtained results indicate that hydrogen evolution overpotential of Pb/Sb/Sn alloy in the presence of ILs increases. This overpotential mainly depends on the concentration of ILs and the number of alkyl and cycloalkyl chains on the cations. Also, the difference between the oxygen oxidation potential in anodic and cathodic scans (∆E) decreases using different ILs.










Similar content being viewed by others
References
Rand DAJ, Moseley PT, Garche J et al (2004) Valve-regulated lead-acid batteries. Elsevier, Amsterdam
Crompton TR (2000) Battery reference book, 3rd edn. Newnes, Oxford, pp 3/1
Berndt D (2001) J Power Sources 95:2
Bui N, Mattesco P, Simon P et al (1997) J Power Sources 67:61
Hibbins SG, Timpano FA, Zuliani DJ (1996) US Patent no. 5:547
Bagshaw NE (1995) J Power Sources 53:25
Rezaei B, Mallakpour S, Taki M (2009) J Power Sources 187:605
Hirasawa T, Sasaki K, Taguchi M et al (2000) J Power Sources 85:44
Rogatchev T, Ruevski ST, Pavlov D (1976) J Appl Electrochem 6:33
Hameenoja E, Hampson NA (1984) J Appl Electrochem 14:449
Garche J, Doring H, Wiesener K (1991) J Power Sources 33:213
Saminathan K, Jayaprakash N, Rajeswari B et al (2006) J Power Sources 160:1410
Welton T (1999) Chem Rev 99:2071
Dupont J, de Souza RF, Suarez PAZ (2002) Chem Rev 102:667
Parvulescu VI, Hardacre C (2007) Chem Rev 107:2615
Arbizzani C, Beninati S, Lazzari M et al (2007) J Power Sources 174:648
Stracke MP, Migliorini MV, Lissner E et al (2009) Appl Energy 86:1512
Ohno H (2005) Electrochemical aspects of ionic liquids. Wiley, New York
Galinski M, Lewandowski A, Stepniak I (2006) Electrochim Acta 51:5567
Hajipour AR, Azizi G, Ruoho AE (2009) Synth Commun 39:242
Caldara F, Delmastro A, Fracchia G et al (1980) J Electrochem Soc 127:1869
Ijomah MNC (1987) J Electrochem Soc 134:2960
Hameenoja E, Laitinen T, Sundholm G et al (1989) Electrochim Acta 34:233
Metikohukovic M, Babic R, Omanovıc S (1994) J Electroanal Chem 374:199
Babic R, Melikos-Hukoric M, Lajqy N et al (1994) J Power Sources 52:17
Guo Y, Wu M, Hua S (1997) J Power Sources 64:65
Brinic S, Metikohukovic M, Babic R (1995) J Power Sources 55:19
Guo Y (1991) J Electrochem Soc 138:1222
Takehara Z (2000) J Power Sources 85:29
Acknowledgements
The authors acknowledge the Isfahan University of Technology Council and Center of Excellency in Sensor and Green Chemistry for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, B., Ensafi, A.A. & Taghipour Jahromi, A.R. Electrochemical performance of lead acid battery using ammonium hydrogen sulphate with different alkyl groups. Ionics 18, 109–116 (2012). https://doi.org/10.1007/s11581-011-0590-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0590-y