Abstract
Objectives
Myocardial strains can be calculated using cardiovascular magnetic resonance (CMR) feature-tracking (FT) algorithms. They show excellent intra- and inter-observer agreement but rather disappointing inter-vendor agreement. Currently, it is unknown how well CMR-FT-based strain values agree with manually obtained strain values.
Methods
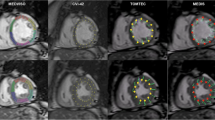
In 45 subjects (15 controls, 15 acute myocardial infarction, 15 non-ischemic dilated cardiomyopathy), end-systolic manually derived strains were compared to four CMR-FT software packages. Global radial strain (GRS), global circumferential strain (GCS) and global longitudinal strain (GLS) were determined. Intra- and inter-observer agreement and agreement between manual and CMR-FT analysis were calculated. Statistical analysis included Bland–Altman plots, intra-class correlation coefficient (ICC) and coefficient of variation (CV).
Results
Manual contouring yielded excellent intra-observer (ICC 0.903 (GRS) to 0.995 (GCS)) and inter-observer agreement (ICC 0.915 (GRS) to 0.966 (GCS)) with CV ranging 4.7% (GCS) to 20.7% (GRS) and 12.7% (GCS) to 20.0% (GRS), for intra-observer and inter-observer agreement, respectively. Agreement between manual and CMR-FT strain values ranged from poor to excellent, with best agreement for GCS (ICC 0.857–0.935) and intermediate for GLS (ICC 0.591–0.914), while ICC values for GRS ranged widely (ICC 0.271–0.851). In particular, two software packages showed a strong trend toward systematic underestimation of myocardial strain in radial and longitudinal direction, correlating poorly to moderately with manual contouring, i.e., GRS (ICC 0.271, CV 25.2%) and GLS (ICC 0.591, CV 17.6%).
Conclusion
Some CMR-FT values agree poorly with manually derived strains, emphasizing to be cautious to use these software packages in the clinical setting. In particular, radial and longitudinal strain tends to be underestimated when using manually derived strains as reference.



Similar content being viewed by others
References
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63:2751–2768
Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 18(1):51
Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S (2016) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37:1196–1207
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680
Hor KN, Baumann R, Pedrizzetti G et al (2011) Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp 48:e2356
Morais P, Marchi A, Bogaert JA et al (2017) Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm. Assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson 19:24
Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, Ferro CJ, Young AA, Townend JN, Leyva F, Steeds RP (2015) Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging 41:1000–1012
Heyde B, Bouchez S, Thieren S et al (2013) Elastic image registration to quantify 3-D regional myocardial deformation from volumetric ultrasound: experimental validation in an animal model. Ultrasound Med Biol 39:1688–1697
Morais P, Heyde B, Barbosa D, Queirós S, Claus P, D’hooge J (2013) Cardiac motion and deformation estimation from tagged MRI sequences using a temporal coherent image registration framework. Springer, Berlin, Heidelberg
Singh A, Steadman CD, Khan JN et al (2015) Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 41:1129–1137
Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S (2016) Cardiovascular magnetic resonance myocardial feature tracking. Concepts and clinical applications. Circ Cardiovasc Imaging 9(4):e0004077
Schuster A, Stahnke V-C, Unterberg-Buchwald C et al (2015) Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol 70:989–998
Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E (2012) Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 14:43
Kuetting DL, Dabir D, Homsi R et al (2016) The effects of extracellular contrast agent (Gadobutrol) on the precision and reproducibility of cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 18(1):30
Kowallick JT, Morton G, Lamata P et al (2016) Inter-study variability of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J Magn Reson Imaging 43:128–137
Aurich M, Keller M, Greiner S et al (2016) Left ventricular mechanics assessed by two-dimensional echocardiography and cardiac magnetic resonance imaging: comparison of high-resolution speckle tracking and feature tracking. Eur Heart J Cardiovasc Imaging 17:1370–1378
Taylor RJ, Moody WE, Umar F et al (2015) Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 16:871–881
Bourfiss M, Vigneault DM, Aliyari Ghasebeh MA et al (2017) Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson 19:66
Barreiro-Pérez M, Curione M, Symons R, Claus P, Voigt JU, Bogaert J (2018) Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking software. Eur Radiol 28:5137–5147
Fleiss JL, Levin B, Paik MC (2003) Statistical methods for rates and proportions, 3rd edn. Wiley, Hoboken
Tao Q, Wenjun Y, Wang Y (2019) Deep-learning-based method for fully automatic quantification of left ventricle function from cine MR images: a multivendor, multicenter study. Radiology 290:81–88
Topol EJ (2019) High-performance medicine: the convergence of human and artificial intelligence. Nat Med 25:44–56
Leiner T, Rueckert D, Suinesiaputra A et al (2019) Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson 21:61
Lamacie MM, Houbois CP, Greiser A, Jolly M, Thavendiranathan P, Wintersperger BJ (2019) Quantification of myocardial deformation by deformable registration-based analysis of cine MRI: validation with tagged CMR. Eur Radiol 29:3658–3668
Kuetting DL, Dabir D, Homsi R et al (2016) The effects of extra-cellular contrast agent (Gadobutrol) on the precision and reproducibility of cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 18:30
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no personal or professional conflicts of interest relating to any aspect of this study.
Ethical standards
This study was approved by the ethical committee of the hospital. Because of the retrospective nature, informed patient consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pierpaolo, P., Rolf, S., Manuel, BP. et al. Left ventricular global myocardial strain assessment: Are CMR feature-tracking algorithms useful in the clinical setting?. Radiol med 125, 444–450 (2020). https://doi.org/10.1007/s11547-020-01159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01159-1