Abstract
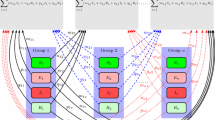
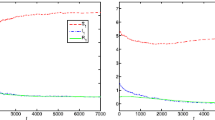
In this paper, we propose control strategies for multigroup epidemic models. We use compartmental \({\textit{SIRS}}\) models to study the dynamics of n host groups sharing the same source of infection in addition to the transmission among members of the same group. In particular, we consider a model for infectious diseases with free-living pathogens in the environment and a metapopulation model with a central patch. We give the detailed derivation of the target reproduction number under three public health interventions and provide the corresponding biological insights. Moreover, using the next-generation approach, we calculate the basic reproduction numbers associated with subsystems of our models and determine algebraic connections to the target reproduction number of the complete model. The analysis presented here illustrates that understanding the topological structure of the infection process and partitioning it into simple cycles is useful to design and evaluate the control strategies.




Similar content being viewed by others
References
Adler B, de la Peña Moctezuma A (2010) Leptospira and leptospirosis. Vet Microbiol 140(3):287–296
Arino J, Portet S (2015) Epidemiological implications of mobility between a large urban centre and smaller satellite cities. J Math Biol 71(5):1243–1265
Arino J, Van den Driessche P (2003) A multi-city epidemic model. Math Popul Stud 10(3):175–193
Baca-Carrasco D, Olmos D, Barradas I (2015) A mathematical model for human and animal leptospirosis. J Biol Syst 23(supp01):S55–S65
Baca-Carrasco D, Velasco-Hernández JX (2016) Sex, mosquitoes and epidemics: an evaluation of zika disease dynamics. Bull Math Biol 78(11):2228–2242
Bani-Yaghoub M, Gautam R, Shuai Z, van den Driessche P, Ivanek R (2012) Reproduction numbers for infections with free-living pathogens growing in the environment. J Biol Dyn 6(2):923–940
Berman A, Shaked-Monderer N (2012) Non-negative matrices and digraphs. In Meyers, R. A. (ed.), Computational complexity: Theory, techniques, and applications. Springer, pp 2082–2095
Chen MI, Ghani AC, Edmunds WJ (2009) A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations. J R Soc Interface 6(38):775–791
Edwards R, Kim S, van den Driessche P (2010) A multigroup model for a heterosexually transmitted disease. Math Biosci 224(2):87–94
Evangelista KV, Coburn J (2010) Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol 5(9):1413–1425
Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM (2006) Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic leptospira. PLoS Med 3(8):e308
Garira W, Mathebula D, Netshikweta R (2014) A mathematical modelling framework for linked within-host and between-host dynamics for infections with free-living pathogens in the environment. Math Biosci 256:58–78
Heesterbeek JAP (2002) A brief history of r0 and a recipe for its calculation. Acta Biotheor 50(3):189–204
Holt J, Davis S, Leirs H (2006) A model of leptospirosis infection in an african rodent to determine risk to humans: seasonal fluctuations and the impact of rodent control. Acta Tropica 99(2):218–225
Horn RA, Johnson CR (2013) Matrix analysis, 2nd. Cambridge University, New York
Keeling M, Tildesley M, House T, Danon L (2013) The mathematics of vaccination. Math Today 49:40–43
Knipl D (2016) A new approach for designing disease intervention strategies in metapopulation models. J Biol Dyn 10(1):71–94
Ko AI, Reis MG, Dourado CMR, Johnson WD, Riley LW, Group SLS (1999) Urban epidemic of severe leptospirosis in Brazil. Lancet 354(9181):820–825
La Salle J, Lefschetz S, Alverson R (1962) Stability by Liapunov’s direct method with applications. Phys Today 15:59
Leirs H, Stenseth NC, Nichols JD, Hines JE, Verhagen R, Verheyen W (1997) Stochastic seasonality and nonlinear density-dependent factors regulate population size in an African rodent. Nature 389(6647):176
Martcheva M (2015) Introduction to mathematical epidemiology, vol 61. Springer, Berlin
Olmos D, Barradas I, Baca-Carrasco D (2017) On the calculation of \(R_0\) using submodels. Differ Equ Dyn Syst 25(3):481–497
Prüss-Üstün A, Corvalán C (2006) Preventing disease through healthy environments. World Health Organization, Geneva
Roberts M, Heesterbeek J (2003) A new method for estimating the effort required to control an infectious disease. Proc R Soc Lond B Biol Sci 270(1522):1359–1364
Shuai Z, Heesterbeek J, van den Driessche P (2013) Extending the type reproduction number to infectious disease control targeting contacts between types. J Math Biol 67(5):1067–1082
Shuai Z, Heesterbeek J, van den Driessche P (2015) Erratum to: extending the type reproduction number to infectious disease control targeting contacts between types. J Math Biol 71(1):255–257
Terpstra W (2003) Human leptospirosis: guidance for diagnosis, surveillance and control. World Health Organization, Geneva
Tien JH, Earn DJ (2010) Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bull Math Biol 72(6):1506–1533
Triampo W, Baowan D, Tang I, Nuttavut N, Wong-Ekkabut J, Doungchawee G (2007) A simple deterministic model for the spread of leptospirosis in Thailand. Int J Biol Med Sci 2:22–26
Ullmann L, Langoni H (2011) Interactions between environment, wild animals and human leptospirosis. J Venom Anim Toxins Incl Trop Dis 17(2):119–129
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1):29–48
Vargas-De-León C (2011) On the global stability of SIS, SIR and SIRS epidemic models with standard incidence. Chaos Solitons Fractals 44(12):1106–1110
Acknowledgements
The authors acknowledge suggestions from an anonymous editor and a referee that helped to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saldaña, F., Barradas, I. Control Strategies in Multigroup Models: The Case of the Star Network Topology. Bull Math Biol 80, 2978–3001 (2018). https://doi.org/10.1007/s11538-018-0503-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0503-6